Article contents
Analysis of in vitro corrosion behavior and hemocompatibility of electrophoretically deposited bioglass–chitosan–iron oxide coating for biomedical applications
Published online by Cambridge University Press: 22 June 2020
Abstract
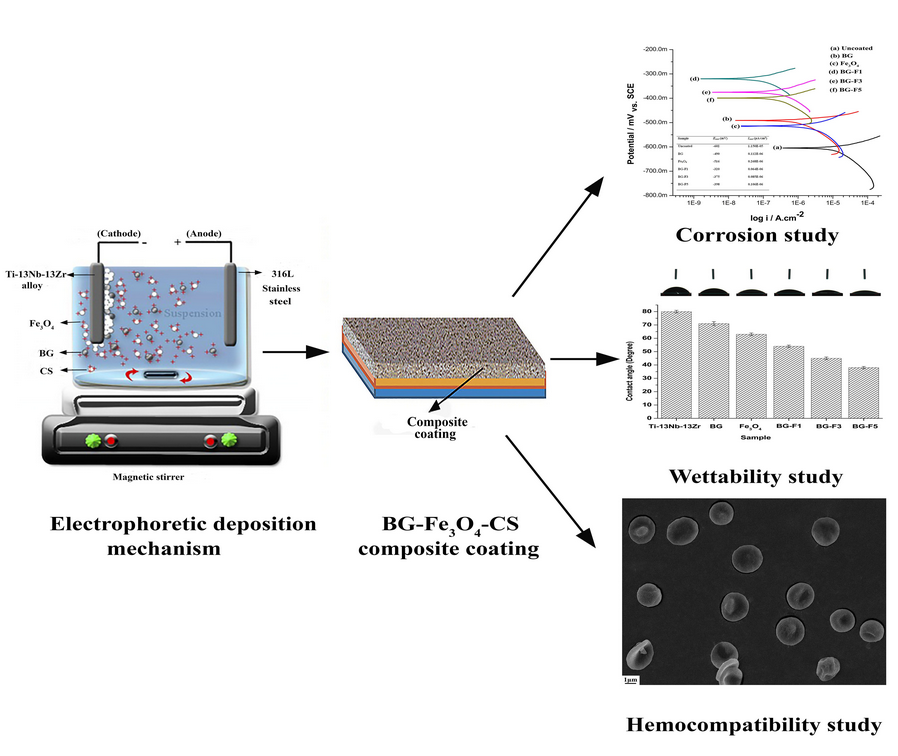
Electrophoretic deposition consisting of bioglass (BG)–chitosan (CS)–iron oxide nanoparticles (Fe3O4 NPs) on the Ti–13Nb–13Zr substrate was described. The bioactive coating was embedded in a CS matrix. The Fe3O4 NPs collected using the co-precipitation method varied at three different levels (1, 3, and 5 wt%) in the BG coating. The formulated coatings exhibited a hydrophilic character due to higher surface roughness values. The pull-off tape test was performed to check the adhesion strength of coatings. The composite coatings displayed adhesion strength of 5B class. The corrosion behavior was evaluated in Ringer's solution by the electrochemical test. The corrosion results showed that the composite coatings were more impressive as compared to pure BG and Fe3O4 coatings. The hemocompatibility results showed a hemolytic ratio (<5%), which validates them as favorable blood compatible nature of the deposited coatings. The findings exhibited that the BG–Fe3O4–CS coating can be widely employed as a favorable material for orthopedic applications.
- Type
- Article
- Information
- Journal of Materials Research , Volume 35 , Issue 13: Focus Section: Interactions of Shear Transformation Bands , 14 July 2020 , pp. 1749 - 1761
- Copyright
- Copyright © Materials Research Society 2020
References
- 6
- Cited by



