Published online by Cambridge University Press: 06 August 2013
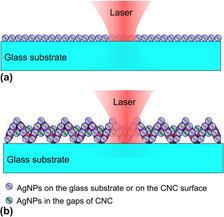
A novel surface enhanced Raman scattering (SERS) substrate was produced by combining Ag nanoparticles (AgNPs) and carbon nanocoils (CNCs). Three different methods were developed for loading AgNPs on CNCs, which include (i) direct deposition of AgNPs on CNCs by radio-frequency magnetron sputtering (RFMS) to form an Ag–CNC hybrid, (ii) deposition of a TiO2 film on CNCs by RFMS, followed by photoinduced growth of AgNPs to form an Ag–TiO2–CNC hybrid (called A-substrate), and (iii) deposition of a TiO2 film on CNCs by spin coating and then photoinduced growth of AgNPs to form an Ag–TiO2–CNC hybrid (called B-substrate). Experimental SERS results showed that B-substrates exhibited the highest SERS enhancement with an enhancement factor of over 107 for rhodamine 6G. The as-prepared Ag–TiO2–CNC substrates also showed much higher Raman signal enhancement than ordinary planar SERS substrates in our system. This was mainly due to the unique three-dimensional structure where the large surface area was available for loading more densely packed AgNPs which contribute to abundant Raman hot spots.