Article contents
Unusual oxidation behavior of light metal hydride by tetrahydrofuran solvent molecules confined in ordered mesoporous carbon
Published online by Cambridge University Press: 20 August 2013
Abstract
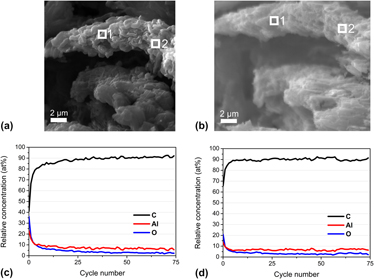
Confining light metal hydrides in micro- or mesoporous scaffolds is considered to be a promising way to overcome the existing challenges for these materials, e.g. their application in hydrogen storage. Different techniques exist which allow us to homogeneously fill pores of a host matrix with the respective hydride, thus yielding well defined composite materials. For this report, the ordered mesoporous carbon CMK-3 was taken as a support for LiAlH4 realized by a solution impregnation method to improve the hydrogen desorption behavior of LiAlH4 by nanoconfinement effects. It is shown that upon heating, LiAlH4 is unusually oxidized by coordinated tetrahydrofuran solvent molecules. The important result of the herein described work is the finding of a final composite containing nanoscale aluminum oxide inside the pores of the CMK-3 carbon host instead of a metal or alloy. This newly observed unusual oxidation behavior has major implications when applying these compounds for the targeted synthesis of homogeneous metal–carbon composite materials.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 29 , Issue 1: Focus Issue: Synthesis of Nanostructured Functional Oxides , 14 January 2014 , pp. 55 - 63
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 2
- Cited by


