Article contents
Synthesis of a novel bioactive glass using the ultrasonic energy assisted hydrothermal method and their biocompatibility evaluation
Published online by Cambridge University Press: 28 August 2014
Abstract
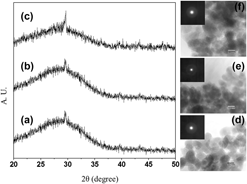
In this study, bioactive glass powders were synthesized via an inexpensive hydrothermal chemical route by the use of ultrasonic energy irradiation. Soda lime, calcium nitrate tetrahydrate, and diammonium hydrogen phosphate were used as the precursor materials to synthesize low-sodium bioactive glass materials based on the conventional 45S5 Bioglass®. The morphological properties of the synthesized powders and the microwave-sintered dense compact were investigated. The mechanical properties of the fabricated dense bioactive glasses were characterized and were found to be very good. The ability to form biological apatite under physiological conditions was demonstrated with simulated body fluid (SBF). The material was shown to be highly biocompatible using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay with osteoblast-like MG63 cells. The cell adhesion and proliferation behavior of osteoblast-like MG63 cells were investigated and found to be excellent.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 4
- Cited by


