Article contents
Synthesis and higher catalytic property of the novel bimetallic Ni–Fe/SiO2 microspheres with mesoporous structure
Published online by Cambridge University Press: 07 February 2017
Abstract
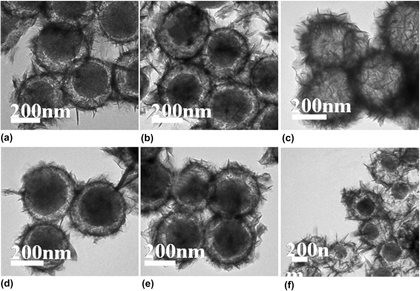
Bimetallic catalyst Ni–Fe/SiO2 microspheres were obtained by reducing bimetallic (Ni,Fe2+)3Si2O5(OH)4 microspheres with controllable morphology structure in situ under the hydrogen atmosphere at 650 °C, which are synthesized via one-step self-template method under hydrothermal conditions. The TEM images indicate the formation process of the different morphology and the synthesis conditions of bimetallic Ni–Fe silicate were obtained. Bimetallic catalyst Ni–Fe/SiO2 (reduced) hollow microspheres had the smaller surface area and the bigger pore diameter than that of (Ni,Fe2+)3Si2O5(OH)4 (unreduced) hollow microspheres because the reduction reaction under high temperature may make part pores in nanosheets collapsing and metal particles aggregating easily for the strong magnetism. For synergistic effect of nickel ion and iron ion, the reaction conditions of the chosen catalyst with higher activity were decreased from 140 °C–24 h + 180 °C −12 h for iron silicate hydroxide to 140 °C–12 h. Ni–Fe/SiO2 core-shell microspheres exhibited excellent catalytic activity with a conversion of nitrobenzene reaching 94% within 2 h, which is 82% higher than Fe/SiO2.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
References
REFERENCES
- 4
- Cited by



