Article contents
Study of orientation relationship between Al matrix and several typical inclusions in Al alloy by edge-to-edge matching model
Published online by Cambridge University Press: 09 April 2017
Abstract
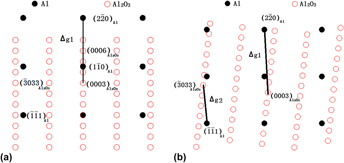
The orientation relationship (OR) between Al matrix and several typical inclusions, Al2O3, MgO, AlN, TiB2, and AlB2, in Al alloy have been studied by edge-to-edge matching model and refined with Δg theory. Based on the calculation of interatomic spacing misfit and interplanar spacing misfit, the number of ORs between Al and Al2O3, MgO, AlN, TiB2, AlB2 were predicted to be 1, 7, 2, 2, and 2, respectively. The result reveals that the wettability of Al to the studied inclusions could rank as MgO, AlB2, TiB2, AlN, Al2O3 in the order of decreasing, and the removability of those particles from aluminum melt rank in an opposite way from the perspectives of crystallography features of interfacial energy. Moreover, Al2O3 have a higher sensitivity to the performance of a processed aluminum alloy component than other inclusions, and MgO has the minimal impact, when the studied inclusions were residual in aluminum alloy.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Jürgen Eckert
References
REFERENCES
- 3
- Cited by



