Published online by Cambridge University Press: 07 May 2019
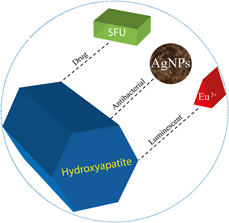
In this work, a multifunctional system was developed in which antibacterial and luminescent properties were inserted into the matrix of hydroxyapatite (HAp) and its efficiency as a support for an antineoplastic drug was evaluated, aiming its application in the treatment of osseous diseases. The precipitation method was used for the synthesis of HAp, EuCl3 was used for the incorporation of Eu3+ as imaging agents, silver nanoparticles (AgNPs) with antimicrobial function were used, and a model of drug, 5-fluorouracil (5FU) was used. The developed material is characterized by several techniques, where crystalline peaks attributed to HAp were identified in the X-ray diffractogram, whereas the luminescence spectrum of the material presented emissions attributed to the Eu3+ ion. The identification and the uniform distribution of AgNPs, 5FU, and Eu3+ were confirmed by mapping the sample using energy-dispersive spectroscopy. The measurements indicated that 82% (±2.8) of 5FU was incorporated into the HAp matrix, and a gradual and increasing release of it as a function of time was observed. Assays carried out for different bacteria confirmed the antimicrobial action of the samples and the efficiency of the drug inserted into the matrix. An in vitro assay showed the bioactivity of the material and its potential to bind to living osseous tissue.