Article contents
Spray pyrolysis of phase pure AgCu particles using organic cosolvents
Published online by Cambridge University Press: 25 September 2013
Abstract
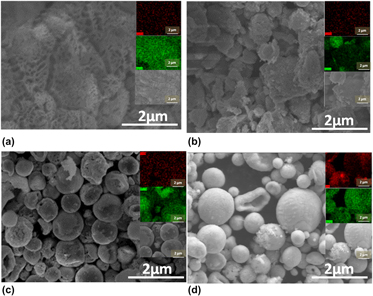
Nano- and micron-sized metal particles have important applications in catalysis and in the medical and electronic industries. For applications requiring high conductivity, such as thick film conductive pastes or isotropic conductive adhesives, AgCu particles combine high conductivity with advantages of lower costs. Here, we report the generation of AgCu particles by spray pyrolysis, a process that has the advantages of simple experimental setup, large-scale production ability, and controllable particle size. Solutions of copper nitrate and silver nitrate dissolved in deionized water with either 40 vol% ethanol (ET) or 40 vol% ethylene glycol (EG) were used as the precursor. Phase separation was observed during the generation of AgCu particles, and the particles were mainly Ag-rich and Cu-rich solid solutions. The short reactor residence time experiments indicated that both the cosolvent properties and operating conditions affect the particle formation process and change the structure of particles.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 8
- Cited by


