Published online by Cambridge University Press: 04 September 2019
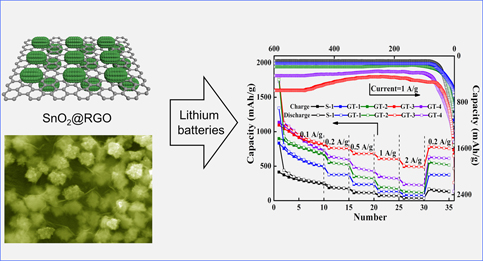
Three-dimensional nano-mulberries consisting of SnO2 nanoparticles have been successfully anchored onto the surfaces of reduced graphene oxide (RGO) to construct hierarchical hybrids—SnO2@RGO with a one-pot approach. The SnO2 nano-mulberries with different amounts of RGO have been produced for optimizing their composition effect on Li storage performance. Specifically, SnO2@RGO hybrids incorporated with optimized amount of RGO nanosheets (∼20.8%) exhibit highly enhanced capacity of ∼1025 mA h/g at 0.1 A/g and a reversible capacity of 750 mA h/g over 100 cycles at 0.2 A/g. The materials also deliver much better rate performance with average specific capacity of ∼498 mA h/g at 2 A/g in comparison with that of SnO2 nano-mulberries. After cycling for 600 times at 1 A/g, the SnO2@RGO electrodes still maintain high reversible capacity of ∼602 mA h/g, corresponding to 35% of the second cycle and 133% of the 70th discharge capacity.