Article contents
Single-pot biofabrication of living fibers for tissue engineering applications
Published online by Cambridge University Press: 01 June 2018
Abstract
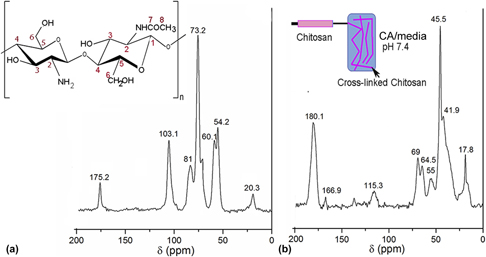
In this work, morphology, viability, and metabolism of the amniotic mesenchymal stem cells conditioned with different citric acid (CA)/media ratios were investigated using rhodamine-phalloidin/4′,6-diamidino-2-phenylindole staining, live/dead assay, proliferating cell nuclear antigen, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL assay). The cells cultured in 25:75 CA/media displayed well spread actin filaments with a prominent nucleus and evidenced optimum viability. The gelation kinetics of chitosan solution in CA/media (25:75) was monitored via dynamic time sweep analysis on a rheometer. The chemical cross-linking of chitosan with CA was confirmed by nuclear magnetic resonance studies. Subsequently, chitosan solution was extruded in CA/media bath containing cells under benign conditions to form cell-laden fibers (living fibers). The prelabeled cells imaged immediately after fiber formation confirmed the attachment of the cells on the fibers. This approach has several advantages including instantaneous gelation, tunable mechanical properties, and adjustable biodegradability that can provide a platform technology for creating viable three dimensional (3D) building blocks for tissue engineering applications.
Keywords
- Type
- Invited Article
- Information
- Journal of Materials Research , Volume 33 , Issue 14: Focus Issue: 3D Printing of Biomaterials , 27 July 2018 , pp. 2019 - 2028
- Copyright
- Copyright © Materials Research Society 2018
Footnotes
These authors contributed equally to this work.
References
REFERENCES
- 2
- Cited by


