Article contents
Oxidation behavior of high-purity nonstoichiometric Ti2AlC powders in flowing air
Published online by Cambridge University Press: 16 March 2017
Abstract
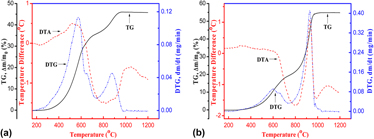
The oxidation behavior of nonstoichiometric Ti2AlC x (x = 0.69) powders synthesized by combustion synthesis was investigated in flowing air by means of simultaneous thermal gravimetric analysis-differential thermal analysis, X-ray diffraction, X-ray photoelectron spectroscopy, and scanning electron microscope/energy dispersive spectroscopy, with an effect of powder size. The oxidation of the fine Ti2AlC powders with the size of about 1 μm starts at 300 °C and completes at 980 °C, while with increasing the powder size around 10 μm the corresponding temperature increases to 400 and 1040 °C, respectively. The oxidation of nonstoichiometric Ti2AlC x (x = 0.69) powders is controlled by surface reaction in 400–600 °C, and mainly diffusion in 600–900 °C, with the corresponding oxidation activation energy of 2.35 eV and 0.12 eV, respectively. In other words, the critical temperature of changing oxidation controlling step is around 600 °C. The oxidation products were mainly rutile-TiO2 and α-Al2O3. The tiny white flocculent particles of α-Al2O3 appeared on the surface of fine Ti2AlC powders and increased with increasing the oxidation temperature.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Yanchun Zhou
References
REFERENCES
- 12
- Cited by



