Article contents
Observation of filament formation process of Cu/HfO2/Pt ReRAM structure by hard x-ray photoelectron spectroscopy under bias operation
Published online by Cambridge University Press: 20 January 2012
Abstract
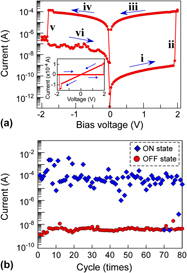
We have demonstrated resistance switching using polycrystalline HfO2 film with a Cu top electrode for nonvolatile memory applications and revealed the Cu diffusion into the HfO2 layer during the filament formation process. Resistive switching was clearly observed in the Cu/HfO2/Pt structure by performing a current–voltage measurement. The current step from a high-resistive state to a low-resistive state was of the order of 103–104 Ω, which provided a sufficient on/off ratio for use as a switching device. The filament formation process was investigated by employing hard x-ray photoelectron spectroscopy under bias operation. The application of a bias to the structure reduced the Cu2O state at the interface and the intensity ratio of Cu 2p3/2/Hf 3d5/2, providing evidence of Cu2O reduction and Cu diffusion into the HfO2 layer. These results also provide evidence that the resistance switching of the Cu/HfO2/Pt structure originates in a solid electrolyte (nanoionics model) containing Cu ions.
- Type
- Invited Feature Paper
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 8
- Cited by


