Article contents
New mechanochemical effects in the poly(N-vinylcaprolactam)—Nano-titanium oxides(IV) system
Published online by Cambridge University Press: 10 April 2018
Abstract
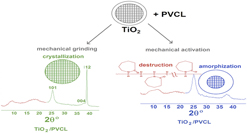
Nanocomposites based on the poly(N-vinylcaprolactam) (PVCL) fabricated from PVCL solutions at different drying temperatures (PVCL25 at 25 °C, PVCL40 at 40 °C) and titanium oxides(IV) nanoparticles (TNPs) were produced for the first time by dry mixing and grinding and mechanical milling in a planetary ball mill using different PVCL:TNP ratios. New effects in initial PVCL (hydration) and TNP [decomposition of η-phase; appearance of hydrated titanium dioxide (HTD)] samples, as well as in PVCL [(de)hydration, disordering of the heterocycles] and TNP (amorphization, dehydration of η-phase, partial crystallization of Hombifine N with anatase), involved as components in PVCL/TNP nanocomposites, were found. The different role of each type of treatments and its conditions in the specific of the effects observed was shown. Only high-frequency mechanical milling leads to the appearance of HTD and the complete disappearance of the second peak of PVCL (disordering of the heterocycles) in PVCL/TNP nanocomposites.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
Footnotes
Contributing Editor: Sarah Morgan
References
REFERENCES
- 6
- Cited by



