Article contents
Neck formation in reactive sintering: A model 2-D experiment
Published online by Cambridge University Press: 13 March 2012
Abstract
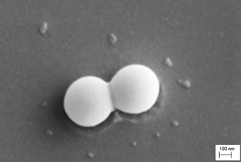
Silica-titania sub-close-packed single layers were deposited by spin coating titanium alkoxide sols containing inert 0.5 micrometer silica particles to study the process of reactive sintering more closely than has been done before. The sub-close-packed single layers were designed to achieve a coating density such that pairs or chains of silica particles were placed on the flat substrate and held together by the reactive titania thin films overlaid on the surface and in the neck regions of these essentially 2-D particle networks. Because of the low density of silica particles, all of the two-particle junctions and neck regions were aligned for geometrically direct viewing; as a result scanning electron microscopy was useful for observing the morphology of these neck regions. Image analysis was used to quantify the neck diameter for varying titania/silica precursor concentration ratios. Geometrical calculations that relate the change in neck volume to the neck radius are presented. Implications for design of reactive sintering systems are discussed.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 2
- Cited by


