Article contents
Nano-tribology studies of reduced graphene oxide films in air and in aqueous solutions with different pH values
Published online by Cambridge University Press: 08 December 2016
Abstract
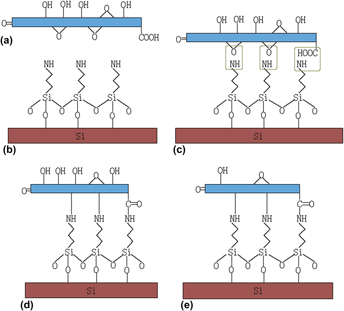
In this study, three types of graphene films—hydrothermal reduced graphene oxide (GO) film, thermal reduced GO film, and GO film—on silicon substrate by using 3-aminopropyl triethoxysilane (APTES) as the interface adhesive layer were prepared for investigation. The chemical compositions of the samples were characterized by x-ray photoelectron spectroscopy (XPS). Surface morphologies, adhesive forces, and nano friction forces in air and aqueous solutions with different pH values were investigated by atomic force microscopy (AFM). Results showed that capillary force dominated the adhesive force in air condition, and adhesive force was much smaller in aqueous solution than in air due to the disappearance of the capillary force. Adhesive force and friction coefficient of the three samples slightly decreased with the increase of pH values. Hydrothermal reduced GO film exhibits the best lubricity both in air and in liquids among those three films.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 5
- Cited by



