Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Wang, N.
Wang, D.P.
Yang, Z.W.
and
Wang, Y.
2016.
Interfacial microstructure and mechanical properties of zirconia ceramic and niobium joints vacuum brazed with two Ag-based active filler metals.
Ceramics International,
Vol. 42,
Issue. 11,
p.
12815.
Yang, Z.W.
Wang, C.L.
Wang, Y.
Zhang, L.X.
Wang, D.P.
and
Feng, J.C.
2017.
Active metal brazing of SiO 2 –BN ceramic and Ti plate with Ag–Cu–Ti + BN composite filler.
Journal of Materials Science & Technology,
Vol. 33,
Issue. 11,
p.
1392.
Wang, N.
Wang, D.P.
Yang, Z.W.
Wang, Y.
and
Liu, X.G.
2017.
Zirconia ceramic and Nb joints brazed with Mo-particle-reinforced Ag-Cu-Ti composite fillers: Interfacial microstructure and formation mechanism.
Ceramics International,
Vol. 43,
Issue. 13,
p.
9636.
Zhang, Qi
Wang, Juan
Zheng, Kaihong
Lu, Yao
and
Yang, Hongwang
2019.
Microstructure evolution and mechanical properties of ZrO2/ZrO2 joints brazed with Ni–Ti filler metal.
Materials Research Express,
Vol. 6,
Issue. 12,
p.
126547.
Ong, Fei Shen
Tobe, Hirobumi
and
Sato, Eiichi
2019.
Intermetallics evolution and fracture behavior of Nb interlayer inserted Si3N4/Ti joints brazed with AgCuTi filler.
Materials Science and Engineering: A,
Vol. 762,
Issue. ,
p.
138096.
Avettand-Fènoël, M.-N.
Naji, K.
and
Pouligny, Ph.
2019.
Brazing vs. diffusion welding of graded Fe based matrix composite and yttria stabilized zirconia.
Journal of Manufacturing Processes,
Vol. 45,
Issue. ,
p.
557.
Chou, Tsung‐Te
Tuan, Wei‐Hsing
and
Lin, Kun‐Lin
2019.
Interface evaluation on the brazed system of AlN‐Ticusil‐Graphite.
International Journal of Applied Ceramic Technology,
Vol. 16,
Issue. 6,
p.
2236.
Li, Lin
Wei, Can
and
Shen, Ping
2020.
Electrochemically driven rapid wetting of 3YSZ by 60Cu–40Ag and its robust joining to 304 stainless steel.
Journal of the European Ceramic Society,
Vol. 40,
Issue. 12,
p.
4281.
Jin, Chenkai
Wang, Ying
Yang, Zhenwen
and
Wang, Dongpo
2020.
C/C composite surface modified by electrophoretic depositing SiC nanowires and its brazing to Nb.
Ceramics International,
Vol. 46,
Issue. 1,
p.
204.
Guo, Weibing
Xue, Haitao
Wei, Xin
and
Zhang, Xiaoming
2020.
Study on the interfacial properties of active Ti element/ZrO2 by using first principle calculation.
International Journal of Applied Ceramic Technology,
Vol. 17,
Issue. 3,
p.
1286.
Fan, Zhou
Wang, Yang
Zhang, Yidong
Yan, Jiazhen
Liu, Jianyi
Tang, Peng
and
Zhang, Qinghui
2021.
First-Principles Investigation on Wetting Interface of Yttrium Stabilized Tetragonal Zirconia Ceramic Filled with AgCuTi Active Filler.
SSRN Electronic Journal ,
Gambaro, S.
Valenza, F.
Muolo, M.L.
Passerone, A.
Riani, P.
and
Cacciamani, G.
2022.
Zirconia-high entropy alloys joints for biomedical applications: The role of Ag-based fillers on interfacial reactivity.
Journal of Alloys and Compounds,
Vol. 909,
Issue. ,
p.
164764.
Qi, Fugong
Wang, Na
Yang, Zhenwen
Wang, Ying
and
Li, Huijun
2022.
Blackening Mechanism and Mechanical Properties Variation of Zirconia Ceramic Induced by Active Metal Brazing.
Advanced Engineering Materials,
Vol. 24,
Issue. 9,
Zhou, Wenlong
Liu, Di
Fu, Wei
Wang, Huaijin
Song, Xiaogong
Hu, Shengpeng
and
Niu, Hongwei
2022.
Insight into the wetting behavior of AgCu‐xTi on ZrO2: Spreading and microstructural evolution.
Journal of the American Ceramic Society,
Vol. 105,
Issue. 10,
p.
6076.
YAO, Yujie
Han, Yong
ZHANG, Lin
Zhang, Kaiyu
Zhu, Haohao
Zhang, Wanliang
Qian, Hangli
and
Zhou, Chengshuang
2022.
The Dependence of Fracture Mode On Interfacial Microstructure in Ta1 Pure Titanium/Agcuni/304 Stainless Steel Vacuum Brazed Joints.
SSRN Electronic Journal ,
LIU, Duo
CHEN, Bin
JIN, Guobiao
SONG, Yanyu
ZHANG, Qi
SONG, Xiaoguo
and
CAO, Jian
2022.
Interfacial characteristics in CNTs-AgCuTi systems.
Chinese Journal of Aeronautics,
Vol. 35,
Issue. 4,
p.
450.
Yao, Yujie
Han, Yong
Zhang, Kaiyu
Zhu, Haohao
Zhang, Wanliang
Qian, Hangli
Zhou, Chengshuang
and
Zhang, Lin
2022.
The dependence of fracture mode on interfacial microstructure in TA1 pure titanium/AgCuNi/304 stainless steel vacuum brazed joints.
Vacuum,
Vol. 203,
Issue. ,
p.
111318.
Habibi, Farzad
and
Samadi, Ahad
2023.
Interfacial reactions in actively brazed Cu-Al2O3 composites and copper using a newly developed Cu-Sn-Ag-Ti filler alloy.
Science and Technology of Welding and Joining,
Vol. 28,
Issue. 6,
p.
444.
Yang, Z.W.
Xiong, Z.
Wang, J.L.
Wang, Y.
and
Wang, D.P.
2023.
Microstructural evolution and high-temperature oxidation resistance of YSZ/Crofer 22H brazed joints using Ag-based filler for solid-oxide fuel cell applications.
Materials Characterization,
Vol. 200,
Issue. ,
p.
112888.
Fischer, Marie
Chaumat, Valérie
Perrière, Loic
and
Hodaj, Fiqiri
2023.
Wetting and interfacial reactions in liquid Au-Ti alloys / ZrO2 system.
Journal of the European Ceramic Society,
Vol. 43,
Issue. 14,
p.
6247.
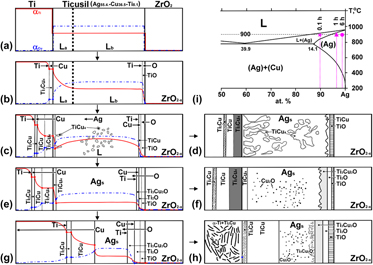
 $\left[ {111} \right]_{{\rm{Cu}}_{\rm{2}} {\rm{O}}} //\left[ {111} \right]_{{\rm{Ag}}}$ and
$\left[ {111} \right]_{{\rm{Cu}}_{\rm{2}} {\rm{O}}} //\left[ {111} \right]_{{\rm{Ag}}}$ and  $\left( {20\bar 2} \right)_{{\rm{Cu}}_{\rm{2}} {\rm{O}}} //\left( {20\bar 2} \right){}_{{\rm{Ag}}}$. After brazing at 900 °C/6 h, a two-phase (α-Ti + Ti2Cu) region was observed on the Ti side with
$\left( {20\bar 2} \right)_{{\rm{Cu}}_{\rm{2}} {\rm{O}}} //\left( {20\bar 2} \right){}_{{\rm{Ag}}}$. After brazing at 900 °C/6 h, a two-phase (α-Ti + Ti2Cu) region was observed on the Ti side with  $\left[ {2\bar 1\bar 10} \right]_{{\rm{\alpha - Ti}}} //\left[ {100} \right]_{{\rm{Ti}}_{\rm{2}} {\rm{Cu}}}$ and
$\left[ {2\bar 1\bar 10} \right]_{{\rm{\alpha - Ti}}} //\left[ {100} \right]_{{\rm{Ti}}_{\rm{2}} {\rm{Cu}}}$ and  $\left( {0002} \right)_{{\rm{\alpha - Ti}}} //\left( {0\bar 13} \right)_{{\rm{Ti}}_{\rm{2}} {\rm{Cu}}}$, while the TiCu layer grew at the expense of Ti3Cu4 and TiCu4. The bonding mechanisms and diffusion paths were explored with the aid of Ag–Cu–Ti and Ti–Cu–O ternary phase diagrams.
$\left( {0002} \right)_{{\rm{\alpha - Ti}}} //\left( {0\bar 13} \right)_{{\rm{Ti}}_{\rm{2}} {\rm{Cu}}}$, while the TiCu layer grew at the expense of Ti3Cu4 and TiCu4. The bonding mechanisms and diffusion paths were explored with the aid of Ag–Cu–Ti and Ti–Cu–O ternary phase diagrams.

