Article contents
Luminescence enhancement of Eu3+-activated La2Mo2O9 red-emitting phosphor through chemical substitution
Published online by Cambridge University Press: 12 September 2011
Abstract
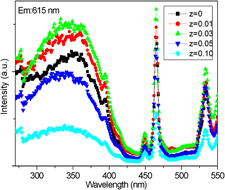
A series of red-emitting phosphors with compositions of La2(Mo1−zSiz)2O9:0.05Eu3+ (0 ≤ z ≤ 0.10) with strong near-UV absorption were prepared by solid-state method. The structure and luminescence properties were investigated by x-ray powder diffraction, UV–vis diffuse reflectivity, and photoluminescence spectra. The luminescent properties as a function of Si4+ concentrations were systematically studied. Under excitation of a wide range near-UV (250–430 nm) or blue light, Si4+-doped series phosphors exhibit characteristic red emission of Eu3+ peaked at 615 nm. The incorporation of Si4+ into La2Mo2O9:0.05Eu3+ phosphor leads to the improvement of the excitation broad band and sharp peaks, as well as the broadening of charge transfer band. Appropriate amount of Si4+ doping can enhance the red luminescence intensity. Finally, the possible reasons for the luminescence enhancement via the corporation of Si4+ were explained.
Keywords
- Type
- Materials Communications
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 5
- Cited by


