Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Liu, B.
Wang, J. Y.
Zhang, J.
Wang, J. M.
Li, F. Z.
and
Zhou, Y. C.
2009.
Theoretical investigation of A-element atom diffusion in Ti2AC (A=Sn, Ga, Cd, In, and Pb).
Applied Physics Letters,
Vol. 94,
Issue. 18,
Yeh, C.L.
and
Kuo, C.W.
2010.
Effects of TiC addition on formation of Ti2SnC by self-propagating combustion of Ti–Sn–C–TiC powder compacts.
Journal of Alloys and Compounds,
Vol. 502,
Issue. 2,
p.
461.
Eklund, Per
Beckers, Manfred
Jansson, Ulf
Högberg, Hans
and
Hultman, Lars
2010.
The M+1AX phases: Materials science and thin-film processing.
Thin Solid Films,
Vol. 518,
Issue. 8,
p.
1851.
Sun, Z M
2011.
Progress in research and development on MAX phases: a family of layered ternary compounds.
International Materials Reviews,
Vol. 56,
Issue. 3,
p.
143.
Abdulkadhim, Ahmed
Takahashi, Tetsuya
Music, Denis
Munnik, Frans
and
Schneider, Jochen M.
2011.
MAX phase formation by intercalation upon annealing of TiC /Al (0.4 ⩽x⩽ 1) bilayer thin films.
Acta Materialia,
Vol. 59,
Issue. 15,
p.
6168.
Kang, Young Jae
Fey, Tobias
and
Greil, Peter
2012.
Synthesis of Ti2SnC MAX Phase by Mechanical Activation and Melt Infiltration.
Advanced Engineering Materials,
Vol. 14,
Issue. 1-2,
p.
85.
Qian, X.K.
2012.
Advances in Science and Technology of Mn+1AXn Phases.
p.
1.
Wang, Xiaohui
Zhang, Hui
Zheng, Liya
Ma, Yonghui
Lu, Xinpo
Sun, Yinjie
Zhou, Yanchun
and
Naslain, R.
2012.
Ti5Al2C3: A New Ternary Carbide Belonging to MAX Phases in the Ti–Al–C System.
Journal of the American Ceramic Society,
Vol. 95,
Issue. 5,
p.
1508.
Shein, I.R.
and
Ivanovskii, A.L.
2012.
Graphene-like titanium carbides and nitrides Tin+1Cn, Tin+1Nn (n=1, 2, and 3) from de-intercalated MAX phases: First-principles probing of their structural, electronic properties and relative stability.
Computational Materials Science,
Vol. 65,
Issue. ,
p.
104.
Qian, Xukun
Wang, Ning
Li, Yuxiang
Zhou, Yuan
Wu, Huaxia
Li, Yibin
and
He, Xiaodong
2012.
First-principle studies of properties of ternary layered M2PbC (M=Ti, Zr and Hf).
Computational Materials Science,
Vol. 65,
Issue. ,
p.
377.
Xie, Yu
and
Kent, P. R. C.
2013.
Hybrid density functional study of structural and electronic properties of functionalized Tin+1Xn(X=C, N) monolayers.
Physical Review B,
Vol. 87,
Issue. 23,
Hu, Chunfeng
Zhang, Haibin
Li, Fangzhi
Huang, Qing
and
Bao, Yiwang
2013.
New phases’ discovery in MAX family.
International Journal of Refractory Metals and Hard Materials,
Vol. 36,
Issue. ,
p.
300.
Ding, Ai Ling
Yu, Wen Bin
Li, Chun Mei
and
Chen, Zhi Qian
2013.
Thermodynamic Properties of Nb<sub>4</sub>AlC<sub>3</sub>.
Applied Mechanics and Materials,
Vol. 456,
Issue. ,
p.
442.
Wang, Jin
Chen, ZhiQian
Li, ChunMei
Li, Feng
and
Nie, ChaoYin
2014.
Electronic structures, elastic properties, and minimum thermal conductivities of cermet M3AlN.
Journal of Solid State Chemistry,
Vol. 216,
Issue. ,
p.
1.
Xu, Hao
Huang, Zhenying
Zhai, Hongxiang
Li, Mengqi
Liu, Xiaohan
and
Zhou, Yang
2015.
Fabrication, Mechanical Properties, and Tribological Behaviors of Ti3Al0.8Sn0.4C2 Solid Solution by Two‐Time Hot‐Pressing Method.
International Journal of Applied Ceramic Technology,
Vol. 12,
Issue. 4,
p.
783.
Zhang, P.
Liu, Y.
Ding, J.
Zhang, Y.M.
Yan, J.L.
An, B.
Iijima, T.
and
Sun, Z.M.
2015.
Controllable growth of Ga wires from Cr2GaC–Ga and its mechanism.
Physica B: Condensed Matter,
Vol. 475,
Issue. ,
p.
90.
Zhang, Peigen
Zhang, Yamei
and
Sun, Zhengming
2015.
Spontaneous Growth of Metal Whiskers on Surfaces of Solids: A Review.
Journal of Materials Science & Technology,
Vol. 31,
Issue. 7,
p.
675.
Khazaei, Mohammad
Arai, Masao
Sasaki, Taizo
Ranjbar, Ahmad
Liang, Yunye
and
Yunoki, Seiji
2015.
OH-terminated two-dimensional transition metal carbides and nitrides as ultralow work function materials.
Physical Review B,
Vol. 92,
Issue. 7,
Bai, Yuelei
He, Xiaodong
and
Wang, Rongguo
2015.
Lattice dynamics of Al‐containing MAX‐phase carbides: a first‐principle study.
Journal of Raman Spectroscopy,
Vol. 46,
Issue. 9,
p.
784.
Huang, Zhenying
Xu, Hao
Zhai, Hongxiang
Wang, Yazheng
and
Zhou, Yang
2015.
Strengthening and tribological surface self-adaptability of Ti3AlC2 by incorporation of Sn to form Ti3Al(Sn)C2 solid solutions.
Ceramics International,
Vol. 41,
Issue. 3,
p.
3701.
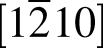 Ti2SnC // [001] Sn. With the combination of DSC and XRD analyses, the precipitation of metallic Sn was demonstrated to be a thermal stress-induced process during the cooling procedure. The reheating temperature, even as low as 400 °C, could trigger the precipitation of Sn from Ti2SnC, which indicated the low-temperature instability of Ti2SnC. A substoichiometry Ti2SnxC formed after depletion of Sn from ternary Ti2SnC phase. Under electron beam irradiation, metallic Sn was observed diffusing back into Ti2SnxC. Furthermore, a new Ti7SnC6 phase with the lattice constants of a = 0.32 and c = 4.1 nm was identified and added in the Ti-Sn-C ternary system.
Ti2SnC // [001] Sn. With the combination of DSC and XRD analyses, the precipitation of metallic Sn was demonstrated to be a thermal stress-induced process during the cooling procedure. The reheating temperature, even as low as 400 °C, could trigger the precipitation of Sn from Ti2SnC, which indicated the low-temperature instability of Ti2SnC. A substoichiometry Ti2SnxC formed after depletion of Sn from ternary Ti2SnC phase. Under electron beam irradiation, metallic Sn was observed diffusing back into Ti2SnxC. Furthermore, a new Ti7SnC6 phase with the lattice constants of a = 0.32 and c = 4.1 nm was identified and added in the Ti-Sn-C ternary system.