Article contents
Indentation experiments and simulation of ovine bone using a viscoelastic-plastic damage model
Published online by Cambridge University Press: 08 November 2011
Abstract
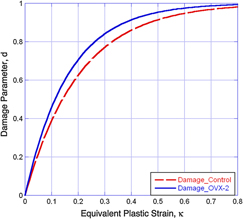
Indentation methods have been widely used to study bone at the micro- and nanoscales. It has been shown that bone exhibits viscoelastic behavior with permanent deformation during indentation. At the same time, damage due to microcracks is induced due to the stresses beneath the indenter tip. In this work, a simplified viscoelastic-plastic damage model was developed to more closely simulate indentation creep data, and the effect of the model parameters on the indentation curve was investigated. Experimentally, baseline and 2-year postovariectomized (OVX-2) ovine (sheep) bone samples were prepared and indented. The damage model was then applied via finite element analysis to simulate the bone indentation data. The mechanical properties of yielding, viscosity, and damage parameter were obtained from the simulations. The results suggest that damage develops more quickly for OVX-2 samples under the same indentation load conditions as the baseline data.
Keywords
- Type
- Articles
- Information
- Journal of Materials Research , Volume 27 , Issue 1: Focus Issue: Instrumented Indentation , 14 January 2012 , pp. 368 - 377
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 2
- Cited by


