Article contents
Heterogeneity in calcium nephrolithiasis: A materials perspective
Published online by Cambridge University Press: 08 May 2017
Abstract
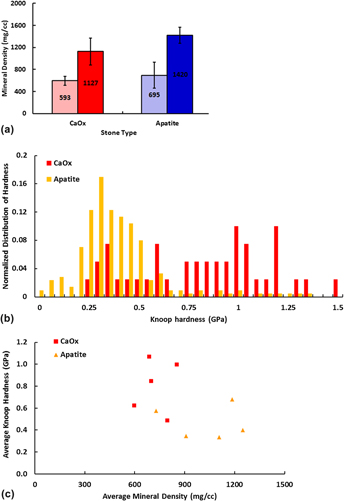
Calcium-based renal calculi demonstrated significant heterogeneity in the structure, density, mineral composition, and material hardness not elucidated by routine clinical testing. Mineral density distributions within calcium oxalate stones revealed differential areas of low (590±80 mg/cc), medium (840±140 mg/cc), and high (1100±200 mg/cc) densities. Apatite stones also contained regions of low (700±200 mg/cc), medium (1100±200 mg/cc), and high (1400±140 mg/cc) densities within layers extending from single or multiple nucleation sites. Despite having lower average mineral density, calcium oxalate (CaOx) stones demonstrated higher material hardness compared to apatite stones, suggesting other chemical components might be involved in determining stone hardness properties. Carbon concentrated sites were identified between morphologic layers in CaOx stones and in stratified layers of apatite stones. Elemental analyses revealed numerous additional trace elements in both stone types. Despite the widespread assumption that stone mineral density is an indicator of susceptibility to lithotripsy, calcium stone mineral density estimates do not directly correlate with actual ex vivo stone hardness. Underlying stone heterogeneity in both structure and mineral density could explain why historical approaches have failed in accurately predicting response of stones to lithotripsy.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Adrian B. Mann
References
REFERENCES
- 6
- Cited by



