Published online by Cambridge University Press: 11 June 2013
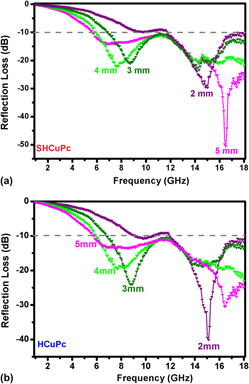
Hyperbranched copper phthalocyanine (CuPc) with uniform spherical morphology has been firstly obtained by ethylene glycol solvothermal synthetic route. The highly dispersed spherical CuPc aggregates with a diameter of ∼500 nm. X-ray diffraction indicated that the molecules were stacked into one-dimensional b-axis aggregate. In addition, the split Soret band together with the broadened and blue-shifted Q-bands in the optical spectra suggested the H (face-to-face) type of interactions in the arrangement of macrocycles in a dense-packed structure. Due to its good symmetrical structure and unique morphology, the hyperbranched spherical CuPc showed excellent broadband microwave absorption behaviors in a frequency of 2–18 GHz. Over an absorber of 5 mm thickness, an absorption bandwidth of 12 GHz corresponding to reflection loss below −10 dB can be obtained. The high value of microwave reflection about −50 dB at the frequency of 16.5 GHz also suggested that the hyperbranched spherical CuPc can be used as promising microwave absorbing materials.