Published online by Cambridge University Press: 29 October 2012
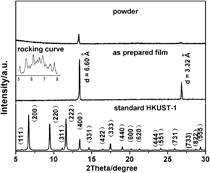
Two-dimensional (2D) disordered copper 1,3,5-tricarboxylate film on copper foil was first reported in this paper. In the x-ray powder diffraction pattern of the as-prepared film, there were only two diffraction peaks exist in d value of 6.60 and 3.32 Å, which were correspond to the (400) and (800) diffractions of bulk HKUST-1, respectively. And the d value of 6.60 Å in bulk HKUST-1 is very close to the thickness of one-layered Cu2+ plus one-layered C9H3O63− (6.59 Å). The structure of as-prepared film was proved to be 2D disordered copper 1,3,5-tricarboxylate film. The periodical stacking of Cu2+ and C9H3O63−is perpendicular to the substrate. There is no periodic structure within the layer. Scanning electron microscopy, transmission electron microscope (TEM), high-resolution transmission electron microscope, infrared, Raman, and x-ray photoelectron spectrometer supported this result. This kind of disorder probably also existed in the reported HKUST-1 film that shows strong (400) diffraction in x-ray diffraction (XRD) pattern. This result probably explains why (400) diffraction in XRD pattern of reported synthesized HKUST-1 film is anomalously strong. This strategy is simple. No seed, no pretreated solvothermal mother liquors, and no specific functionalization are required.