Published online by Cambridge University Press: 07 November 2019
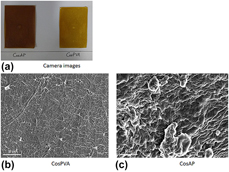
Fabrication of simulated buccal mucosa could minimize sacrificing the animals (rabbits and pigs) to extract buccal mucosa for in vitro testing of buccal formulations. Novel artificial buccal mucosa was fabricated using eggshell membrane, extracted from poultry egg, and bovine submaxillary mucin. Chitosan oligosaccharide (COS)–based blended films were fabricated using solvent casting technique. Patches of equal dimensions were cut precisely from whole film. COS-based blended patches were analyzed for their physicochemical and mechanical properties. These patches, proposed to be used for buccal drug delivery, were tested for their mucoadhesion timing using the artificial mucosal membrane. The COS–PVA–blended patch displayed better mucoadhesion than chitosan oligosaccharide–alginate–blended film with the fabricated simulated buccal mucosa. Novel buccal mucosa mimetic–surface such as the one reported in this research article could prove to be a very useful tool in minimizing the use of excised animal buccal mucosa for mucoadhesion testing of buccal drug delivery formulations. Novel COS-blended films were fabricated as a proposed mucoadhesive buccal drug delivery vehicle.