Article contents
Enhanced photocatalytic activity of TiO2–niobate nanosheet composites
Published online by Cambridge University Press: 19 November 2012
Abstract
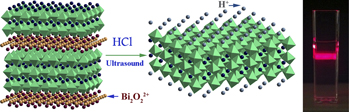
Protonated niobate nanosheets, H1.8Bi0.2CaNaNb3O10 (BCNN), were synthesized using a new organic-free simultaneous ion exchange and exfoliation process from the Aurivillius phase Bi2CaNaNb3O12. Nanosheet/TiO2 composites were prepared by thermal treatment of physical mixtures of commercially available anatase TiO2 and the nanosheet suspension. Methylene blue (MB) dye degradation studies for the composite show a clear correlation between the MB surface adsorption and the degradation rate. The composite exhibits strongly enhanced photocatalytic activity as the calcination temperature increases, suggesting the possibility of charge transfer at the BCNN–TiO2 interface and the existence of Nb5+ and O2−acid–base pairs. Both phenomena are attributed to the processing approach, which includes topochemical dehydration of the BCNN nanosheets during heat treatment.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 28 , Issue 3: Focus Issue: Titanium Dioxide Nanomaterials , 14 February 2013 , pp. 424 - 430
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 10
- Cited by


