Published online by Cambridge University Press: 13 January 2016
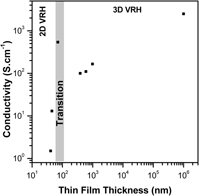
In the present work, we are investigating the electronic transport mechanism for antimony-doped tin oxide (ATO) ultrathin films produced by a colloidal deposition process (CDP) of nanocrystals synthesized via a solvothermal route in organic medium. The ATO ultrathin films were prepared from nanoparticles containing 9 mol% of Sb and the observed electrical resistivity at room temperature was 1.55, 1.10 × 10−1, and 1.83 × 10−3 Ω cm, respectively, for the 40, 45, and 71 nm films. X-ray diffraction, transmission electron microscopy, scanning electron microscopy, and atomic force microscopy were carried out to investigate the films and electrical resistivity measurements taken in the four-probe mode with temperature ranging from −260 to 27 °C (13–300 K ± 0.1 K). Results show a good data fitting on Mott's two-dimensional (2D) noninteracting variable range hopping for the 45 nm thin film, which is not further observed for the ATO ultrathin films obtained from CDP.