Article contents
Effects of position, thickness, and annealing temperature of Ag buffer layer on the shape of ZnO nanocrystals grown by a simple hydrothermal process
Published online by Cambridge University Press: 16 December 2013
Abstract
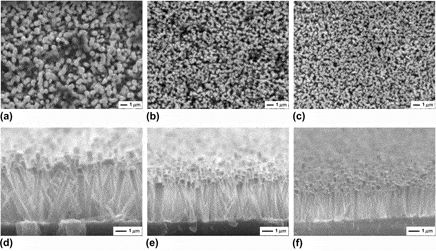
In this paper, we report on the well-aligned zinc oxide (ZnO) nanorods synthesized on Ag buffer layer/glass substrate using a modified hydrothermal method, which adopts the strategy of Ag layer facing down. The effects of position, thickness, and annealing temperature of Ag layer on the shape of ZnO nanocrystals were systematically investigated. It was found that the diameter and length of ZnO nanorods decrease with the Ag layer height up to 12 mm, above which no obvious decrease was observed. Oppositely, the density, diameter, and length of ZnO rods all increase with an increase in the Ag layer thickness, except that the length becomes constant above a critical thickness of 60 nm. In addition, when the Ag layer annealing temperature increases from 300 to 400 °C, the nanorod density decreases, the diameter increases, and the length remains nearly invariable, respectively. Surprisingly, randomly inclined nanorods with two different diameters dispersedly coexist on the Ag layer that was annealed at 500 °C. This work may provide an effective approach for the shape control in ZnO-based applications.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 1
- Cited by


