Article contents
Effects of polymer chemistry, concentration, and pH on doxorubicin release kinetics from hydroxyapatite-PCL-PLGA composite
Published online by Cambridge University Press: 20 May 2019
Abstract
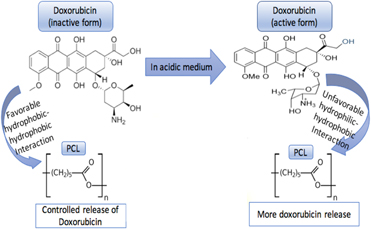
The objective of this study was to understand the effects of ceramic polymer composite and pH of the surrounding vicinity on the release kinetics of doxorubicin. Different concentrations of polymers with polycaprolactone (PCL), poly glycolic lactic acid (PLGA), and a blend of PCL–PLGA with hydroxyapatite (HA) were investigated for doxorubicin release at physiological pH of 7.4 and an acidic pH of 5.0 caused by immediate surgery. Burst release of 20% was observed from bare HA at pH 7.4 over a week, whereas all the polymer incorporated discs showed sustained release. The hydrophilic–hydrophobic and hydrophobic–hydrophobic interactions between the polymer and the drug altered by the surrounding pHs were found to be pivotal in controlling the release kinetics of drug. No cytotoxicity of the drug at a concentration of 50 μg per disc was observed at early time points when cultured with osteoblast cells; however, the same drug dosage inhibited osteosarcoma cell viability. This study mainly bases on the comprehension of the effects of chemistry, environment, and polymer–drug interactions, leading to a beneficial understanding towards the design of drug delivery devices.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
Footnotes
This author was an editor of this journal during the review and decision stage. For the JMR policy on review and publication of manuscripts authored by editors, please refer to http://www.mrs.org/editor-manuscripts/.
References
- 5
- Cited by


