Article contents
Effects of biomimetic micropattern on titanium deposited with PDA/Cu and nitric oxide release on behaviors of ECs
Published online by Cambridge University Press: 20 May 2019
Abstract
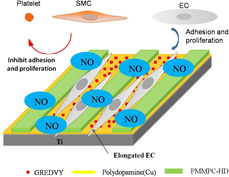
Surface modification with poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) is an effective method for improving hemocompatibility. Peptide GREDVY immobilized on Ti is of great benefit to endothelialization. Micropattern of PMPC and GREDVY can regulate cells distribution, behaviors, and nitric oxide (NO) release. Copper can be used as catalytic to release NO from a donor in vitro, which can inhibit platelets adhesion, activation, and aggregation. The Ti-PDA(Cu)-M/R(P) micropattern was fabricated with PMMPC-HD {PMMPC [monomer contain MPC and methacrylic acid (MA)] was cross-linked with hexamethylene diamine} and peptide Gly-Arg-Glu-Asp-Val-Tyr (GREDVY) using PDMS stamp, and it was characterized by SEM, FTIR, and XPS. The results demonstrated that the PMPC and peptide GREDVY were immobilized onto polydopamine successfully. Simultaneously, the copper existed in polydopamine was confirmed by XPS. The rate of NO release in vitro catalyzed by copper ions was 1.5–5.3 × 10−10 mol/(cm2 min). It will be beneficial to inhibiting platelets adhesion and proliferation of ECs.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 9
- Cited by


