Article contents
Effect of aging on the corrosion behavior of 6005 Al alloys in 3.5 wt% NaCl aqueous solution
Published online by Cambridge University Press: 15 May 2018
Abstract
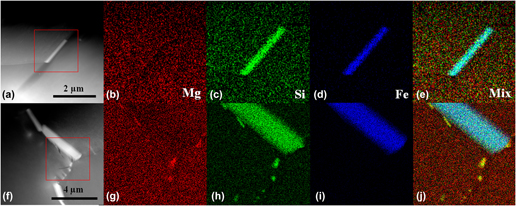
The effect of aging time on the corrosion behavior of 6005 Al alloys has been investigated in aerated 3.5% NaCl aqueous solution. The corrosion resistance of the alloy with different aging times is analyzed by measuring potentiodynamic polarization, electrochemical impedance spectroscopy. The surface morphology is examined by scanning electron microscopy. The results demonstrated that the corrosion resistance of the alloy in the peak-aged condition is worse than the other conditions. Accordingly, corrosion rate and the corrosion current density of the alloy reach its maximum value. An Fe-rich phase is identified as the β-Al4.5FeSi phase by atomic-resolution high angle annular dark field scanning transmission electron microscopy and energy dispersive X-ray spectroscopy mapping analyses. The β-Al4.5FeSi is wrapped slowly by the precipitates of Mg2Si from the process of the peak-aged condition to the over-aged condition. It is hypothesized that the change of corrosion behavior of the alloy may be attributed to the β-Al4.5FeSi wrapped slowly by the precipitates of Mg2Si.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 9
- Cited by



