Article contents
Dynamic responses of reactive metallic structures under thermal and mechanical ignitions
Published online by Cambridge University Press: 28 September 2012
Abstract
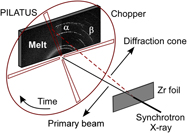
We have studied dynamic thermo-mechano-chemical responses of reactive metallic systems, both in clouds of small oxygen-free particles (∼1–10 μm in diameter) produced by fracturing Zr-rich bulk metallic glass and in pure Zr metal foils (∼25 μm thin), under thermal (laser ablation or pulse electrical heating) and mechanical loadings. The mechanical fracture/fragmentation and fragments reactions were time resolved using an integrated set of fast six-channel optical pyrometer, high-speed microphotographic camera, and time- and angle-resolved synchrotron x-ray diffraction. These small-scale tabletop real-time experiments performed on or near surfaces of reactive metals provide fundamental data, in atomistic scales or of particle clouds, regarding fragmentation mechanics, combustion mechanisms and kinetics, and dynamics of energy release under thermal and mechanical loadings. We present the results of pure Zr and Zr-rich amorphous metals, not only signifying diversified combustion mechanisms depending on microstructures, particle sizes, oxygen pressure, and ignition conditions but also providing fundamental data that can be used to develop and validate thermochemical and mechanochemical models for reactive materials.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 11
- Cited by


