Article contents
Dopant-controlled photoluminescence of Ag-doped Zn–In–S nanocrystals
Published online by Cambridge University Press: 28 June 2017
Abstract
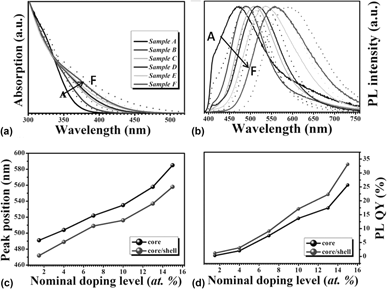
In this work, we reported the growth of cadmium-free Ag-doped Zn–In–S nanocrystals (NCs) with effective photoluminescence (PL) via a hot-injection strategy. The effects of the nucleation temperatures, reaction times, and Ag-doping concentrations on the PL properties of Ag-doped Zn–In–S NCs were investigated systematically. The as-synthesized NCs exhibit color-tunable PL emissions covering a broad visible range of 472–585 nm. After being passivated by a protective ZnS shell, the PL quantum yield (QY) of the resultant NCs was greatly improved up to 33%. With the increase of the Ag-doping level, the PL is significantly intensified due to the improved concentration of Ag ions which provides more holes to recombine with electrons from the bottom of the conduction band. This also makes the emission via the dopant energy level become a powerful, competitive advantage for the NCs with higher Ag-doping levels, resulting in a longer lifetime and higher PL QY. These results suggest that tailoring the Ag-doping level can be a powerful strategy to control the optical properties of Ag-doped Zn–In–S NCs.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Winston V. Schoenfeld
References
REFERENCES
- 4
- Cited by



