No CrossRef data available.
Article contents
Differential scanning calorimetric measurements and thermodynamic functions of YbRhO3(s)
Published online by Cambridge University Press: 31 October 2018
Abstract
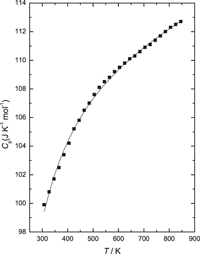
Standard molar heat capacity,  $C_{{\rm{p,m}}}^{\rm{o}}\left( T \right)$, of YbRhO3(s) was determined using a heat flux type differential scanning calorimeter from 126 to 846 K. The heat capacities in the temperature range 307 ≤ T (K) ≤ 846 were fitted into a polynomial expression and can be represented by
$C_{{\rm{p,m}}}^{\rm{o}}\left( T \right)$, of YbRhO3(s) was determined using a heat flux type differential scanning calorimeter from 126 to 846 K. The heat capacities in the temperature range 307 ≤ T (K) ≤ 846 were fitted into a polynomial expression and can be represented by  $C_{{\rm{p,m}}}^{\rm{o}}$ (YbRhO3, s, T) [J/(K mol)] = 106.82 + 8.57 × 10−3T (K) − 9.48 × 105/T2(K). The standard molar heat capacity of YbRhO3(s),
$C_{{\rm{p,m}}}^{\rm{o}}$ (YbRhO3, s, T) [J/(K mol)] = 106.82 + 8.57 × 10−3T (K) − 9.48 × 105/T2(K). The standard molar heat capacity of YbRhO3(s),  $C_{{\rm{p,m}}}^{\rm{o}}$, at 298.15 K is 98.7 J/(K mol). The standard molar Gibbs energy of formation of YbRhO3(s) was also determined using calcia stabilized zirconia as an oxide electrolyte and air as the reference electrode by the solid state electrochemical technique. The cell can be represented by (−)Pt–Rh/{Yb2O3(s) + YbRhO3(s) + Rh(s)}//CSZ//O2(p(O2) = 21.21 kPa)/Pt–Rh(+). The electromotive force was measured in the temperature range from 889 to 1110 K. The standard Gibbs energy of formation of YbRhO3(s) from elements in their standard state can be represented by ΔfGo{YbRhO3(s)}/kJ/mol(±1.62) = −1110.9 + 0.287 T (K). The heat capacity of YbRhO3(s) was used along with the data obtained from the electrochemical cell to evaluate all thermodynamic functions of YbRhO3(s).
$C_{{\rm{p,m}}}^{\rm{o}}$, at 298.15 K is 98.7 J/(K mol). The standard molar Gibbs energy of formation of YbRhO3(s) was also determined using calcia stabilized zirconia as an oxide electrolyte and air as the reference electrode by the solid state electrochemical technique. The cell can be represented by (−)Pt–Rh/{Yb2O3(s) + YbRhO3(s) + Rh(s)}//CSZ//O2(p(O2) = 21.21 kPa)/Pt–Rh(+). The electromotive force was measured in the temperature range from 889 to 1110 K. The standard Gibbs energy of formation of YbRhO3(s) from elements in their standard state can be represented by ΔfGo{YbRhO3(s)}/kJ/mol(±1.62) = −1110.9 + 0.287 T (K). The heat capacity of YbRhO3(s) was used along with the data obtained from the electrochemical cell to evaluate all thermodynamic functions of YbRhO3(s).
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018


