Article contents
Development of sandwich-structured cobalt porphyrin/niobium molybdate nanosheets catalyst for oxygen reduction
Published online by Cambridge University Press: 15 November 2018
Abstract
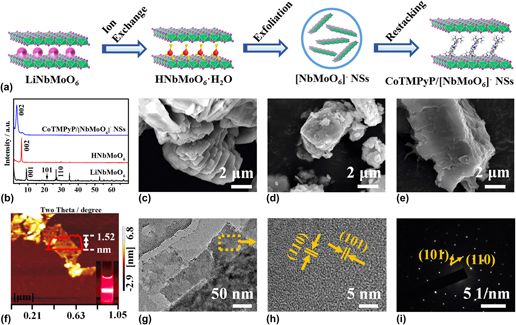
In this work, the negatively charged [NbMoO6]− nanosheets (NSs) were combined with positively charged [5,10,15,20-tetrakis (N-methylpyridinium-4-yl) porphyrinato cobalt] (CoTMPyP) to fabricate a sandwich-like CoTMPyP/[NbMoO6]− NSs intercalated material by a direct self-assembling process. The results confirmed that CoTMPyP cations formed an inclined monolayer between [NbMoO6]− NSs and the inclined angle was about 68°. The electrochemical properties of CoTMPyP/[NbMoO6]− NSs composite were also investigated by cyclic voltammetry and liner sweep voltammetry, which showed the enhanced electron transferred ability. The CoTMPyP/[NbMoO6]− NSs modified electrode displayed excellent electrocatalytic activity towards oxygen reduction with the reduction peak potential shifting from −0.681 to −0.235 V. And oxygen could be reduced to generate hydrogen peroxide with a two-electron process in neutral electrolytes. Moreover, the reduction peak current was linear relationship with the square root of scan rates, implying that the catalytic reaction depended on oxygen diffusion.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 4
- Cited by


