Article contents
An X-ray scattering and electron microscopy study of methylammonium bismuth perovskites for solar cell applications
Published online by Cambridge University Press: 10 January 2017
Abstract
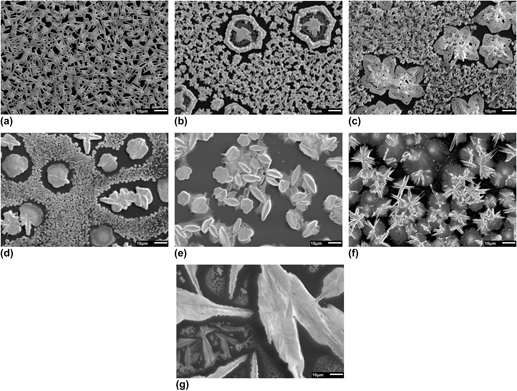
Photovoltaics made from organic–inorganic hybrid perovskite semiconductors are attracting significant interest due to their ability to harvest sunlight with remarkable efficiency. The presence of lead in the best performing devices raises concerns regarding their toxicity, a problem that may create barriers to commercialization. Hybrid perovskites with reduced lead content are being investigated to overcome this issue and here we evaluate bismuth as a possible lead substitute. For a series of hybrid perovskite films with the general composition CH3NH3(Pb y Bi1−y )I3−x Cl x , we characterize their optical and structural properties using UV–Vis spectroscopy, scanning electron microscopy and grazing incidence wide angle X-ray scattering. We show that they form crystalline structures with an optical band gap, around 2 eV for CH3NH3BiI3. However, preliminary solar cell tests show low power conversion efficiencies (<0.01%) due to both incomplete precursor conversion and material de-wetting from the substrate. The overall outcome is severely limited photocurrent. With current processing methods the general applicability of hybrid bismuth perovskites in photovoltaics may be limited.
- Type
- Invited Articles
- Information
- Journal of Materials Research , Volume 32 , Issue 10: Focus Issue: Microstructural Characterization for Emerging Photovoltaic Materials , 26 May 2017 , pp. 1888 - 1898
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Chris Nicklin
References
REFERENCES
- 5
- Cited by



