Introduction
Nasopharyngeal carcinoma is a distinct clinical entity which is endemic in the southern Chinese population but rare in Caucasians. In Singapore, it is the commonest head and neck cancer, with age-standardised incidences in males and females of 10.8 and 3.7 per 100 000 per year, respectively.Reference Seow, Koh, Chia, Shi, Lee and Shanmugaratnam1 This radiosensitive tumour is usually treated with radiotherapy.Reference Heng, Wee, Fong, Lian, Sethi and Chua2, Reference Lee, Law, Foo, Poon, Cheung and Chan3 In recent years, concurrent chemoradiotherapy has been used to treat advanced locoregional disease, with better survival results.Reference Al-Sarraf, Leblanc, Giri, Fu, Cooper and Vuong4, Reference Wee, Tan, Tai, Wong, Leong and Tan5
After completion of their definitive treatment regimes, and with complete clinical response of the primary nasopharyngeal carcinoma, some patients have residual cervical lymphadenopathy. Such lymphadenopathy may harbour disease or may merely represent post-treatment necrosis or hyaline fibrosis, without viable tumour cells. There is a paucity of data addressing the management of this clinical problem. Furthermore, the best method of determining the presence of persistent disease in this group of patients is currently not known. The treatment of persistent cervical nodal disease after definitive neck treatment in patients with nasopharyngeal carcinoma is controversial; studies have often combined this patient group with patients presenting with recurrent nodal disease. Some centres have advocated repeated radiotherapy for the treatment of such persistent cervical lymphadenopathy, while others have recommended surgery.Reference Ho, Wei, Sham, Lau and Lam6–Reference Perez, Ackerman, Mill, Ogura and Powers11
Previously, we have published data on the use of fine needle aspiration cytology (FNAC) and computed tomography (CT) scanning in assessing recurrent cervical nodal disease, together with data on the incidence of negative neck dissection in cases of recurrent nodal disease.Reference Toh, Yuen, Goh and Goh12 We believe that residual cervical lymphadenopathy represents a distinct clinical challenge and should be addressed separately.
We postulate that, in patients with nasopharyngeal carcinoma who present with residual cervical lymphadenopathy after completion of a definitive treatment regime, such lymphadenopathy may not necessary represent persistent nodal disease. In this study, we aimed (a) to investigate the diagnostic value of FNAC and CT scanning in such patients, and (2) to determine the incidence of negative neck dissection in this clinical group. We hope that our results will clarify the management of this select group of patients.
Methods and patients
This was a retrospective clinical review of patients who had undergone curative treatment for nasopharyngeal carcinoma and who subsequently presented with residual cervical lymphadenopathy to the Otolaryngology and Head and Neck surgery department of Singapore General Hospital over an 11-year period (April 1995 to December 2006). Twelve such patients were identified and their clinical, radiological, surgical and histological records reviewed. Patients with histologically proven residual disease at the primary site were excluded from the study.
All 12 patients received either a standard course of conventional radiotherapy or concurrent chemoradiotherapy, and all patients completed their treatment protocols. Patients who received standard radiotherapy received 70 Gy in 35 fractions, 2 Gy per fraction. The neck was treated with 60 Gy, and lymph nodes were boosted with electrons for another 10 Gy. All fields were treated once daily, five times a week. Patients who were treated with chemoradiotherapy received concurrent cisplatin administered during weeks one, four and seven of radiotherapy, plus further adjuvant cisplatin and fluorouracil chemotherapy between weeks 11 and 19.Reference Wee, Tan, Tai, Wong, Leong and Tan5 Patients underwent a standardised post-treatment surveillance protocol involving both the otolaryngologist and radiotherapist. In the first year post-treatment, all patients underwent monthly reviews to detect residual disease at the primary site and cervical lymph nodes.
‘Residual or persistent nodal disease’ was defined as persistence of lymphadenopathy, without complete remission, after completion of the entire treatment regime.Reference Ho, Wei, Sham, Lau and Lam6 In the group of patients in question, there is currently no standardised time-frame for observation of lymphadenopathy prior to investigation or treatment for possible persistent nodal disease. In our institution, we perform a CT scan and FNAC if the patient has residual, clinically palpable lymphadenopathy three months after completion of definitive treatment.
We assessed the diagnostic value of FNAC and CT in determining residual nodal disease in this clinical setting. All patients with clinically palpable residual cervical nodes underwent standard FNAC. Briefly, aspiration of cells from palpable lymph nodes was performed using a 23-gauge needle mounted on a 10-ml syringe, making 10–15 passes in multiple directions. Both air-dried and wet-fixed slides were prepared, and the adequacy of the specimen was checked by a trained technician. The slides were reviewed by fellowship-trained pathologists who were part of the multidisciplinary head and neck tumour team. The FNAC result was defined as ‘positive’ if malignant cells with nuclear pleomorphism and scanty cytoplasm with ill-defined cytoplasmic borders were seen (Figure 1), ‘negative’ if no malignant cells were seen (Figure 2), and ‘inconclusive’ if atypical cells were seen which were suspicious for malignancy but inconclusive (Figure 3). We compared the FNAC findings with results for final histological examination of surgically resected lymph node specimens. We then calculated the sensitivity, specificity, and positive and negative predictive values of FNAC in assessing persistent nodal disease.

Fig. 1 Photomicrograph of fine needle aspirate from residual cervical lymph node persisting after definitive treatment, showing malignant cells. (Diff Quik (DQ); ×200)

Fig. 2 Photomicrograph of fine needle aspirate from a residual cervical lymph node persisting after definitive treatment, showing neutrophils and lymphocytes on a background of red blood cells. (Diff Quik (DQ); ×400)

Fig. 3 Photomicrograph of fine needle aspirate from a residual cervical lymph node persisting after definitive treatment, showing a cluster of atypical cells. (Diff Quik (DQ); ×600)
Computed tomography findings were also correlated with the final histopathological results. A CT scan was considered positive for pathological lymphadenopathy based on the following criteria: the presence of nodal necrosis, and/or the presence of lymph nodes with a greatest diameter of more than 10 mm.Reference Brown and Fee13–Reference Zhang, Liang, Li, Cai, Chen and Cai15
Results
During the review period, 12 patients presented with suspected persistent nodal disease after completing their respective treatment regimes. All patients had World Health Organization type 2a or 2b nasopharyngeal carcinoma at primary diagnosis.
The demographics of the study population are shown in Table I.
Table I Characteristics of study population
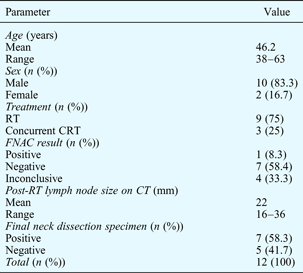
RT = radiotherapy; CRT = chemoradiotherapy; FNAC = fine needle aspiration cytology; CT = computed tomography
All 12 patients had clinically and radiologically evident pathological residual cervical lymphadenopathy suggestive of persistent disease. All patients had previously had a complete response of the primary tumour to treatment, both clinically and radiologically. Figure 4 shows a CT scan of the pre-irradiated neck of a patient with nasopharyngeal carcinoma and left cervical lymph node enlargement. Figure 5 shows a CT scan of the same patient three months after treatment, which fulfils the criteria for pathological lymphadenopathy. Fine needle aspiration cytology was negative for malignancy. This patient underwent radical neck dissection for suspected persistent disease; final histopathological examination showed hyalinised necrosis but no viable tumour cells (Figure 6).
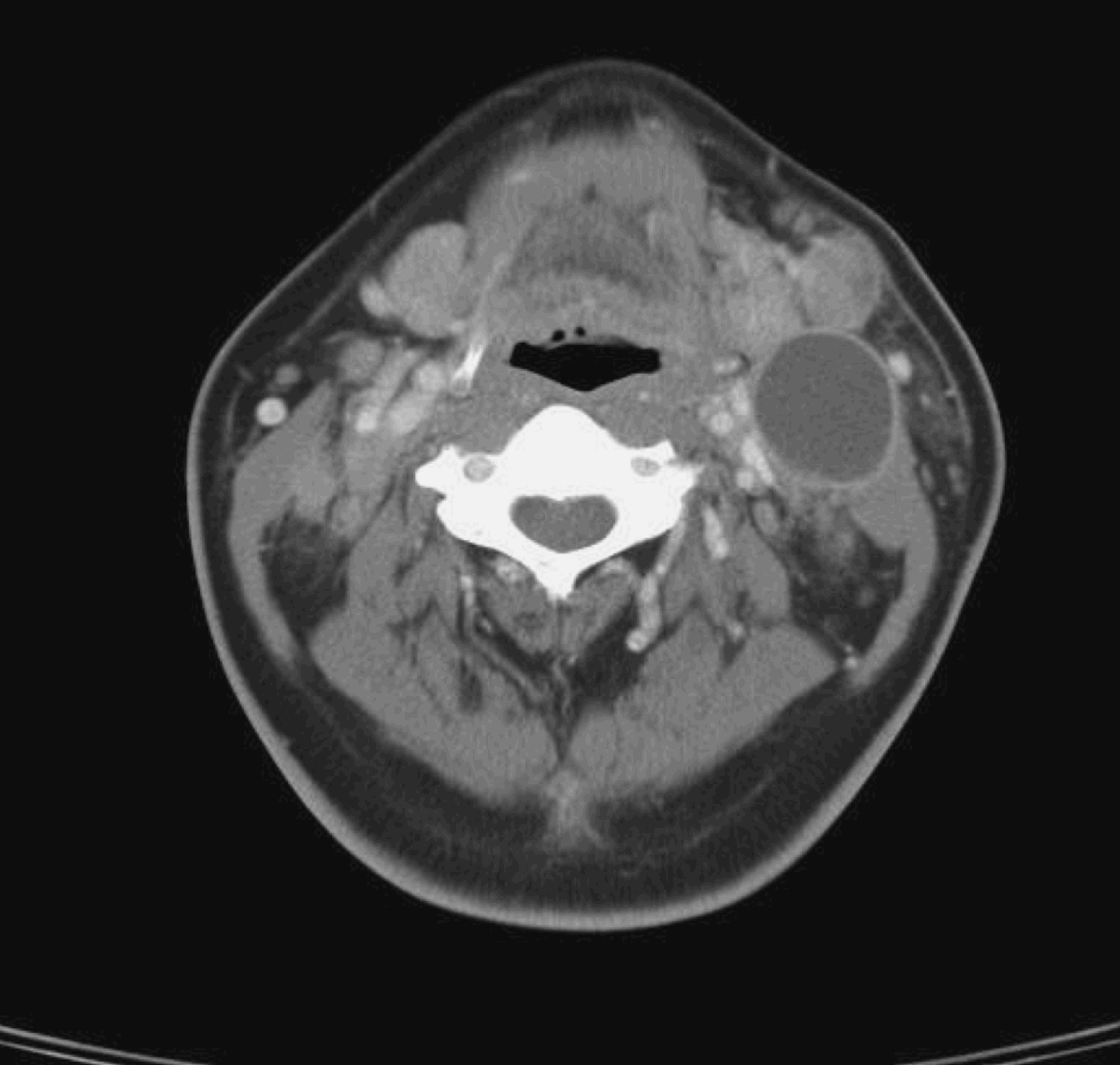
Fig. 4 Axial computed tomography neck scan with intravenous contrast, for a patient with untreated nasopharyngeal carcinoma and cervical nodal disease.

Fig. 5 Axial computed tomography scan with intravenous contrast for the same patient as in Figure 4, after definitive treatment; cervical lymphadenopathy is reduced in size but not completely resolved.

Fig. 6 Photomicrograph of a large, 3.6-cm, necrotic cervical lymph node taken from the same patient as in Figure 4 following definitive treatment, showing hyalinised necrosis with no viable tumour cells. A rim of benign lymphoid cells is seen on the right. (H&E; ×100)
Fine needle aspiration cytology was performed for all 12 patients. One of these examinations (8.3 per cent) was positive for malignant cells, seven (58.4 per cent) were negative for malignancy, and four (33.4 per cent) contained atypical cells which were suspicious for malignancy but inconclusive.
These FNAC results were correlated with the results of histological examination of neck dissection specimens. Seven (58.3 per cent) of the 12 patients had a positive result for histological examination of the final neck specimen, while the other five (41.7 per cent) had a negative result. The sensitivity, specificity and diagnostic predictive values of FNAC were calculated for the eight patients with a definite (either positive or negative) FNAC report, and are shown in Table II. Three (75 per cent) of the four patients with a suspicious FNAC result had a positive neck dissection specimen result.
Table II Sensitivity/ Specificity/ Positive and negative predictive value of FNAC in assessing residual cervical nodal lymphadenopathy for disease

All 12 patients received a radical or modified radical neck dissection: nine underwent unilateral radical neck dissection and three bilateral synchronous neck dissection (i.e. a radical neck dissection on one side and a contralateral modified radical neck dissection sparing the internal jugular vein).
Five (41.7 per cent) of the 12 patients had no viable tumour cells in the resected lymph nodes; nodes showing tumour necrosis or hyalinisation (Figures 7 and 8).

Fig. 7 Photomicrograph of a 2-cm residual cervical lymph node after definitive treatment for nasopharyngeal carcinoma, showing hyalinisation of lymph node with peripheral residual normal lymphocytes. (H&E; ×200)

Fig. 8 Photomicrograph of another patient's hyalinised lymph node, with no malignant cells seen. (H&E; ×100)
The other seven (58.3 per cent) patients had positive neck dissections with lymph node involvement on more than one level, although their CT scans showed only single level involvement. Two patients had level one nodal involvement, all seven patients had level two nodal involvement and three patients had level three nodal involvement. Figure 9 shows a pre-treatment CT neck scan of a patient with bilateral cervical nodal disease. Three months after completion of treatment, this patient had residual left level two cervical lymphadenopathy with central necrosis (Figure 10). Fine needle aspiration cytology of the lymph node showed atypical cells. The patient underwent radical neck dissection. The final histopathological specimen was positive and had more nodal disease than was suggested by the CT scan.

Fig. 9 Pre-treatment axial computed tomography scan with intravenous contrast, showing bilateral cervical nodal disease in a patient with nasopharyngeal carcinoma.
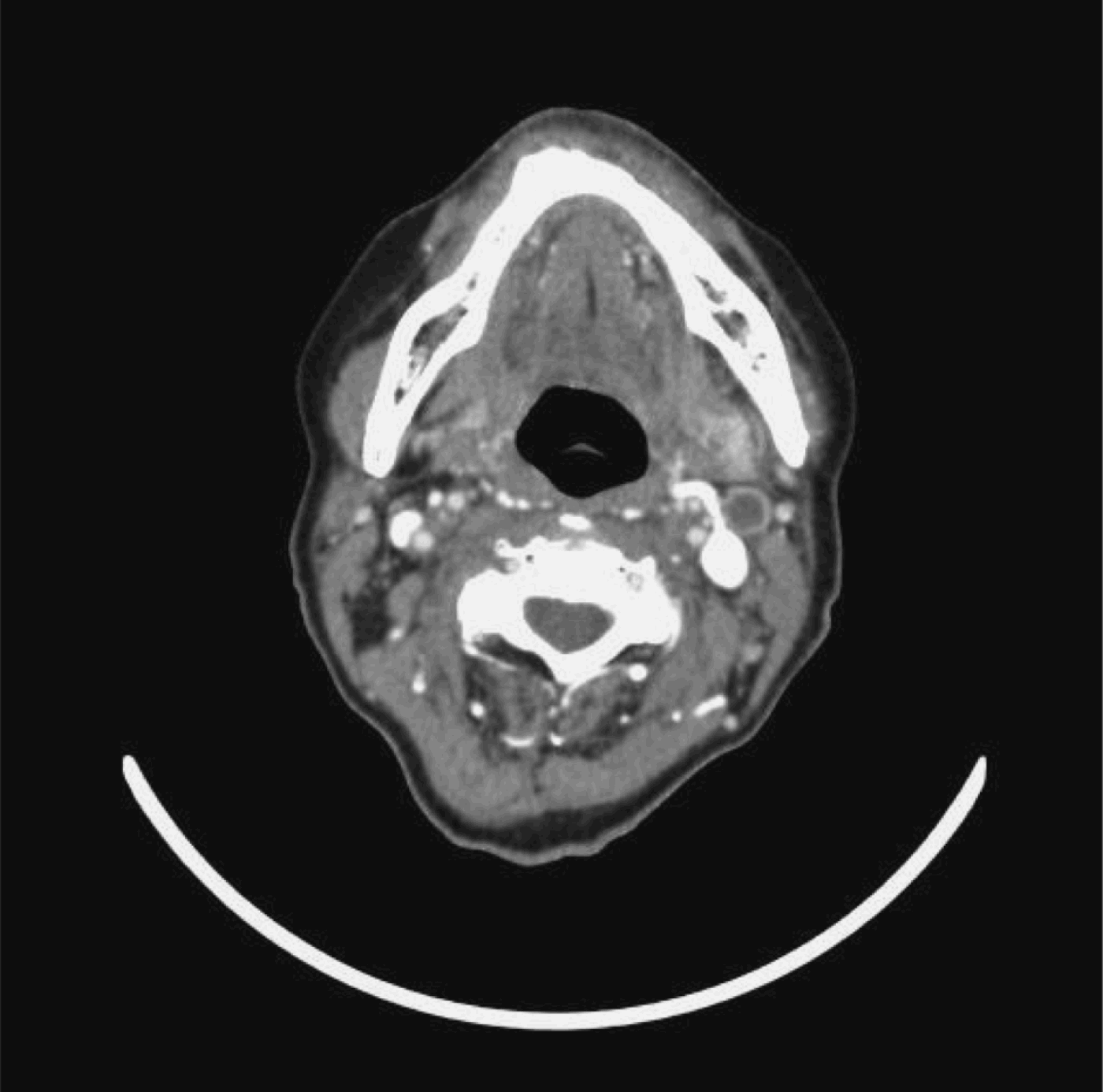
Fig. 10 Post-treatment axial computed tomography scan with intravenous contrast, for the same patient as in Figure 9, showing residual left cervical lymph node with central necrosis.
Discussion
Our study results indicate that residual cervical lymphadenopathy after definitive treatment for nasopharyngeal carcinoma does not necessarily represent persistent disease. In five (41.7 per cent) of our 12 patients, the final histopathological neck dissection specimen did not show viable tumour cells.
Residual cervical lymphadenopathy after curative radiotherapy or chemoradiotherapy for nasopharyngeal carcinoma is a challenging clinical problem. The need for treatment of residual lymphadenopathy, and the timing and best modality of such treatment, are still controversial issues. Most published studies have combined this patient group with patients with recurrent nodal disease.Reference Ho, Wei, Sham, Lau and Lam6–Reference Wei, Lam, Ho, Sham and Lau9 In our clinical practice, however, we encountered a substantial number of negative neck dissections in this group of patients, and felt that such cases should be addressed separately; we therefore undertook the present study.
Ho et al. studied patients undergoing radical neck dissection for suspected persistent cervical nodal disease, and found tumour cells in all their patients' histological specimens (i.e. the negative neck dissection rate was 0 per cent).Reference Ho, Wei, Sham, Lau and Lam6 However, Chen and Fletcher did not find any viable tumour cells in the radical neck dissection specimens of their six patients whose nodes did not resolve after radiotherapy (a negative neck dissection rate of 100 per cent). Perez et al. reported that only one out of seven patients undergoing neck dissections for suspected persistent nodal disease had histological specimens containing tumour cells (a negative neck dissection rate of 85.7 per cent).Reference Chen and Fletcher10, Reference Perez, Ackerman, Mill, Ogura and Powers11 Wei et al. reported that one out of five patients with suspected persistent nodal disease did not show any tumour cells in their neck dissection specimen (a 20 per cent negative neck dissection rate), of 109 lymph node specimens.Reference Wei, Lam, Ho, Sham and Lau9 In the patient with negative neck dissection, the authors showed that the 2 cm persistent lymph node showed reactive hyaline fibrosis.
In our study of patients with suspected persistent cervical nodal disease after curative treatment for nasopharyngeal carcinoma, the incidence of negative neck dissection, from final histological specimens, was 41.7 per cent (five out of 12 patients). In such negative neck dissection cases, the final histological specimen showed either necrosis without viable tumour cells (Figure 6) or hyalinisation of the nodes (Figure 7). Our patients' negative neck dissection rate was higher that that of patients with recurrent nodal disease. The negative neck dissection rate for patients with recurrent nodal disease following nasopharyngeal carcinoma treatment is well established. In a separately published study, we reported a 10.8 per cent negative neck dissection rate for 42 patients presenting with recurrent nodal disease.Reference Toh, Yuen, Goh and Goh12 Other studies combining patients with residual and recurrent disease in their analysis have reported negative neck dissection rates of 4–12 per cent.Reference Wei, Wai, Cheng, Wu, Li and Nicholls8, Reference Wei, Lam, Ho, Sham and Lau9
There is no standardised time-frame for observing residual lymph nodes before instituting treatment. Perez et al. reported waiting at least four weeks before performing radical neck dissection for seven patients with suspected persistent nodal disease.Reference Perez, Ackerman, Mill, Ogura and Powers11 Yen et al. studied four patients with suspected persistent nodal disease, and reported waiting for two consecutive months to allow a more objective assessment of the disease state of the neck, before performing salvage neck dissection at nine to 10 weeks.Reference Yen, Hsu, Sheen, Chang and Hsu7 Wei et al. reported waiting three months after completion of radiotherapy before undertaking salvage surgery in five patients with persistent nodal disease.Reference Wei, Wai, Cheng, Wu, Li and Nicholls8 Other authors have not stated how long they would observe residual lymphadenopathy before instituting treatment.Reference Ho, Wei, Sham, Lau and Lam6, Reference Wei, Lam, Ho, Sham and Lau9 In our department, we wait three months before investigating residual lymphadenopathy.
There is currently no well accepted method for pre-operative determination of the presence of persistent cervical lymph node malignancy. Furthermore, there is little in the literature documenting the efficacy of FNAC or CT in assessing this group of patients. We undertook this study to determine the sensitivity, specificity and diagnostic accuracy of FNAC and CT scanning in positively identifying true persistent nodal disease.
We currently believe that if the CT scan shows pathological lymphadenopathy, the nodal basin should be cleared. However, we routinely perform FNAC of cervical lymphadenopathy in patients with suspected persistent nodal disease. In this clinical setting, we are aware that pre-operative FNAC confirmation of residual nodal disease may not always be possible; firstly, it may not be possible to obtain an adequate cytological specimen, and secondly, the interpretation of the FNAC result may be difficult due to post-irradiation necrosis and neck fibrosis.Reference Wei, Wai, Cheng, Wu, Li and Nicholls8, Reference Wei, Lam, Ho, Sham and Lau9 However, FNAC remains the best method of pre-operative cytological diagnosis of cervical lymphadenopathy under these circumstances, and enables better counselling of patients regarding treatment options. For clinically useful FNAC with a definitive result (either positive or negative), we calculated a sensitivity of 25 per cent, a specificity of 100 per cent, a positive predictive value of 100 per cent and a negative predictive value of 42.9 per cent. We also noted that if atypical cells were seen on the FNAC specimen and reported as suspicious but indeterminate or inconclusive, there was a high probability of finding viable tumour cells in the final neck dissection specimen. Three of our four patients whose FNAC was reported as suspicious were found to have positive nodal disease on histological examination of the neck dissection specimen. There are no data in the literature addressing the sensitivity and specificity of FNAC in this clinical setting. There are however some studies addressing the sensitivity and specificity of FNAC in patients with suspected recurrent nodal disease following nasopharyngeal carcinoma treatment, and also comparable data for patients with residual and recurrent disease analysed together. Yen et al. reported a sensitivity of 83 per cent for FNAC performed for 12 patients with suspected recurrent nodal disease.Reference Yen, Hsu, Sheen, Chang and Hsu7 Wei et al. found that 20 of 33 FNAC samples from patients with residual and recurrent nodal disease showed malignant cells, while histopathological examination found tumour cells in 45 of 51 patients' neck dissection specimens.Reference Wei, Lam, Ho, Sham and Lau9 In a separate study, we published data on 42 patients presenting with suspected recurrent cervical nodal disease; we found a sensitivity, specificity, positive predictive value and negative predictive value of 75, 75, 93.8 and 37.5 per cent, respectively, for the use of FNAC to identify disease.Reference Toh, Yuen, Goh and Goh12 Rates as low as 57 per cent have been reported for the sensitivity of FNAC to detect recurrent nodal disease.Reference Yen, Hsu, Sheen, Chang and Hsu7 Whether FNAC is needed in the first place, or should be repeated if negative, is controversial. In our department, if the CT scan shows pathological lymphadenopathy we will undertake treatment regardless of the FNAC result.
Computed tomography is an important follow-up tool in the management of patients with residual cervical lymphadenopathy after nasopharyngeal carcinoma treatment, but its utility in identifying true persistent disease may be limited. In our study, although all our 12 patients with residual cervical lymphadenopathy fulfilled the criteria for pathological nodal disease, only seven (58.3 per cent) had positive disease on final examination of neck dissection specimens. A similar result has been reported for patients with recurrent nodal disease. In our previously published study on recurrent nodal disease, CT scanning had a positive predictive value of 78.6 per cent and a negative predictive value of 20 per cent for multilevel lymph node involvement.Reference Toh, Yuen, Goh and Goh12 The final pathological neck dissection specimen showed more nodal involvement than was radiologically evident.Reference Toh, Yuen, Goh and Goh12 These findings mirror reports by other investigators.Reference Ho, Wei, Sham, Lau and Lam6, Reference Wei, Lam, Ho, Sham and Lau9, Reference Ho, Chan, Tsao and Li16, Reference Wei, Ho, Wong, Ng, Lau and Lam17
High resolution ultrasound (US) is reported to be more sensitive than clinical examination (92 per cent versus 70 per cent) in detecting and differentiating benign from malignant nodes.Reference Bruneton, Roux, Caramella, Demard, Vallicioni and Chauvel18 Furthermore, the specificity of US combined with guided FNAC has been reported to be as high as 92.7 per cent.Reference Baatenberg de Jong, Rongen, De Jong, Lameris, Harthoorn and Verwoerd19 However, Ahuja and colleagues' study of patients with nasopharyngeal carcinoma found that, for up to three months after radiotherapy, apart from a decrease in node size and the appearance of oedema, other features of previously abnormal nodes (i.e. reflectivity, nodal border, intranodal necrosis and absence of hilus) remained fixed.Reference Ahuja, Yim, Leung and Metreweli20 It was therefore not possible to use any of the features studied to distinguish nodes that might contain residual disease. The same authors stated that US-guided FNAC is not useful after radiotherapy (although FNAC is well documented to significantly increase the specificity of US). Following radiotherapy, it is often more difficult to obtain positive FNAC samples from the neck nodes, and the cytological interpretation of the aspirate is also more difficult. This is particularly true in the early post-treatment period as tumour cells are not uniformly viable. In our institution, we do not perform US-guided FNAC on such patients.
The reported treatment for residual cervical lymphadenopathy varies from additional boosts of radiotherapy, through excision biopsy with post-operative irradiation if specimens are positive for persistent disease, to radical neck dissection.Reference Ho, Wei, Sham, Lau and Lam6–Reference Wei, Lam, Ho, Sham and Lau9, Reference Ho, Chan, Tsao and Li16, Reference Wei, Ho, Wong, Ng, Lau and Lam17, Reference Tu, Hu, Xu and Ye21, Reference Yan, Hu and Gu22 Those authors who selected radical neck dissection as the surgical treatment for residual cervical lymphadenopathy did so based on pathological studies which found that, in cases of persistent and recurrent nodal disease, more tumour-harbouring lymph nodes were detected in the final neck dissection specimen than in prior clinical or radiological examinations.Reference Ho, Wei, Sham, Lau and Lam6–Reference Wei, Lam, Ho, Sham and Lau9, Reference Ho, Chan, Tsao and Li16, Reference Wei, Ho, Wong, Ng, Lau and Lam17, Reference Yan, Hu and Gu22
These same pathological studies also found extracapsular and isolated tumour cell clusters in the neck dissection specimens.Reference Ho, Wei, Sham, Lau and Lam6–Reference Wei, Lam, Ho, Sham and Lau9, Reference Ho, Chan, Tsao and Li16, Reference Wei, Ho, Wong, Ng, Lau and Lam17, Reference Yan, Hu and Gu22 Our study found greater numbers and more levels of nodal involvement than were clinically or radiologically evident; we also detected the presence of extracapsular spread. Our seven (58.3 per cent) patients with a positive neck dissection had lymph node involvement in more than one level, although their CT scans only showed single level involvement. Two patients had level one nodal involvement, all seven patients had level two nodal involvement and three patients had level three nodal involvement. This finding is consistent with the results of other studies.Reference Ho, Wei, Sham, Lau and Lam6, Reference Wei, Lam, Ho, Sham and Lau9, Reference Ho, Chan, Tsao and Li16–Reference Wei, Ho, Wong, Ng, Lau and Lam17
• Patients with nasopharyngeal carcinoma who undergo definitive treatment to the primary site and regional lymph nodes may still have residual cervical lymphadenopathy
• Such cervical lymphadenopathy may not represent persistent disease
• In the same patient group, nodal necrosis seen on computed tomography may also not represent persistent disease
• If such patients are scheduled to undergo radical neck dissection, they should be counselled pre-operatively regarding the possibility of a negative result
The role of positron emission tomography (PET) in detecting persistent disease is controversial, and this imaging modality has yet to become the standard of care because of its prohibitive cost.Reference Yen, Hong, Tzen, Pan and Chen23, Reference Ng, Joseph, Chan, Ko, Wang and Liao24
While the efficacy and safety of radical neck dissection in treating this group of patients is well documented, such surgery may be unnecessary for those without proven disease. Our study found that five of our 12 patients with persistent cervical lymphadenopathy may not need a full radical neck dissection. Therefore, we propose that patients who present with residual cervical lymphadenopathy after curative radiotherapy or concurrent chemoradiation of nasopharyngeal carcinoma, and whose FNAC is negative or suspicious, should undergo an excision biopsy with intra-operative frozen section, and should proceed to neck dissection if tumour cells are found.Reference Brown and Fee13 In the irradiated neck, it is generally recommended to avoid pre-operative open biopsies as there is a direct correlation between wound edge necrosis and pre-operative open biopsy.Reference Ho, Wei, Sham, Lau and Lam6 Alternatively, patients could undergo a PET scan. If the PET scan shows residual disease, the patient should undergo radical neck dissection. However, if the PET scan shows no residual disease, the surgeon may elect either to follow the patient closely, or to perform an excision biopsy with intra-operative frozen section and then proceed to radical neck dissection if the frozen section shows viable tumour cells. The role of PET scanning and excision biopsy in this clinical situation needs further study.
The present study was conducted on patients with nasopharyngeal carcinoma who had undergone curative radiotherapy or chemoradiotherapy but who had suspected persistent cervical nodal disease. Therefore, our reported CT, FNAC and final dissection specimen findings relate only to these 12 patients. The role of FNAC and CT in the evaluation of patients who have received neck radiotherapy for other head and neck cancers should be assessed separately.
Conclusion
Residual cervical lymphadenopathy persisting after curative treatment of nasopharyngeal carcinoma is a challenging clinical problem for the head and neck surgeon. While the efficacy of radical neck dissection is well documented, it may not be necessary in all such patients. Our study found that 41.7 per cent of such patients did not harbour viable tumour cells. In the absence of a diagnostic investigation proven to identify persistent nodal disease, our patients were treated with radical neck dissection. Our study showed that FNAC has good specificity and a high positive predictive value but a poor negative predictive value for identification of disease. Computed tomography was highly sensitive but had a poor positive predictive value. We recommend that head and neck surgeons consider a PET scan or an excision biopsy with intra-operative frozen section to confirm the presence of disease, before proceeding with neck dissection. Patients should be counselled on the possibility of a negative neck dissection.














