Introduction
Laryngeal cancer is diagnosed in approximately 2400 people every year in the UK.1 Treatment options include transoral laser microsurgery, transoral robotic surgery and open partial laryngeal surgery for early-stage disease (stage I/II).
In the UK, transoral laser microsurgery using the carbon dioxide (CO2) laser, when surgical access permits its use, is a cost-effective treatment modality when compared to radiotherapy,Reference Prettyjohns, Winter, Kerawala, Paleri and ;2 with comparable disease-free and laryngeal preservation rates.Reference Elicin and Giger3 The laser has a wavelength of 10 600 nm, and a high co-efficient of extinction in water, which limits its penetration and collateral effects.Reference Shankar, Chakravarthi and Shilpakar4 It provides an ideal and precise cutting instrument for laryngeal lesions.Reference Polanyi, Bredemeier and Davis5
The vocal folds of the human larynx are composed of five histological layers, each with a specific function. Preservation of these layers, while also ensuring adequate cancer clearance, presents a dilemma to the surgeon. Unnecessary damage to healthy tissues should be minimised to reduce the effects on functional outcomes such as voice.Reference Bocca, Pignataro and Mosciaro6 A clear surgical margin, normally defined as greater than 1.00 mm, indicates the absence of cancer cells at the resection margin at the microscopic level. A close margin can be considered to be 1.00 mm or less.Reference Alicandri-Ciufelli, Bonali, Piccinini, Marra, Ghidini and Cunsolo7 Positive margins require further treatment; close margins may require further treatment depending on tumour characteristics.Reference Ansarin, Santoro, Cattaneo, Massaro, Calabrese and Giugliano8
Heat causes tissue shrinkage associated with collagen contracture at temperatures above 50°C.Reference Wall, Deng, Torzilli, Doty, O'Brien and Warren9 Transoral laser microsurgery is a thermally mediated treatment, hence shrinkage of the resected laryngeal specimen is expected.Reference Rossmann, Garrett-Mayer, Rattay and Haemmerich10 The thermal effects from the laser can have detrimental effects on the surgical mucosal margin, which can shrink after excision.Reference Mistry, Qureshi and Kumaran11 If the surgical margins shrink after excision, even by only 1–2 mm, it can be difficult for the histopathologist to accurately report the margins to determine whether or not they are involved. This can result in difficulties in deciding whether or not the patient undergoes revision surgery or adjuvant treatment, such as radiotherapy, that may be unnecessary.
Following resection, some surgeons use alcohol-dried cucumber to mount the specimen, providing the histopathologist with an orientation of the resected laryngeal specimen.Reference Murray, Cooper, Handa, MacLeod and MacKenzie12 It is possible that this process may further alter the size of the specimen, and therefore affect the interpretation of the surgical margins.
The current study aimed to quantify the amount of shrinkage of a type II laryngeal excision specimen,Reference Remacle, Eckel, Antonelli, Brasnu, Chevalier and Friedrich13 comparing shrinkage between cold steel resection (control condition) and CO2 laser resection, and to determine the additional effects of mounting the specimen on cucumber.
Materials and methods
Cadaveric material
Harvested fresh-frozen human larynxes were removed using the same operating technique. The larynxes were stored in a freezer between −18°C and −21°C.
Cucumber preparation
For mounting resected specimens onto cucumber, a 10-blade scalpel was used to cut slices of cucumber measuring 5 mm thick with digital callipers. A triangular shaped section was cut from the centre of each slice. The cucumber slices were submerged into an airtight container with 99 per cent isopropyl alcohol, which was replaced every 24 hours for 3 consecutive days. The final slices were stored in absolute alcohol until required, at which point they were dried with a paper towel to remove excess fluid.Reference Murray, Cooper, Handa, MacLeod and MacKenzie12
Dissection protocol
The frozen larynxes were defrosted by submerging them into warm water for 15 minutes before use. They were dried with surgical gauze. A midline incision was made in the posterior aspect of each larynx to improve access. The larynx was placed on a flat board, covered in damp surgical gauze. A self-retaining retractor was used to keep the larynx open (Figure 1). A 10 mm ruler was held using non-toothed forceps on the true vocal fold and then false vocal fold. The CO2 laser was used to mark the top, middle and bottom of the resection, using the ruler as a marker for all specimens. A European Laryngological Society type II laryngeal resection was performed between these marks.Reference Remacle, Eckel, Antonelli, Brasnu, Chevalier and Friedrich13
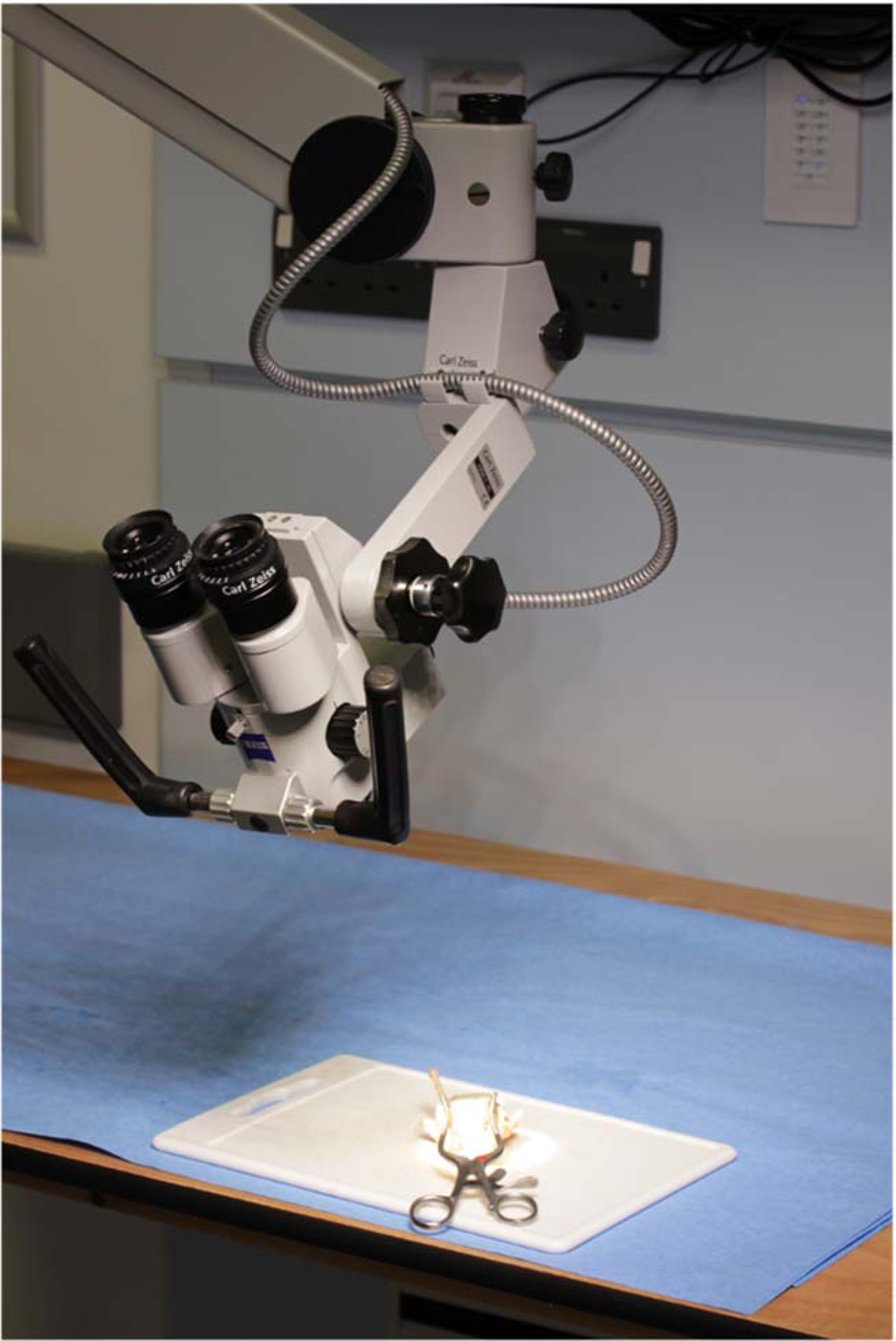
Fig. 1. Photograph demonstrating set-up for the dissection. The larynx was positioned on the cutting board and held in position with a self-retaining retractor.
Laser resection was performed on the right side of each specimen and, as a control, cold steel resection was performed on the left side. Resections were all performed by the senior author (AG) using the Zeiss OPMI® 1FC surgical microscope on an S21 floor stand. The CO2 laser used was the SmartXideReference Prettyjohns, Winter, Kerawala, Paleri and ;2 laser system (model C60; JenaSurgical, Jena, Germany) (1.0 W, dot-shaped ultra-pulse mode, continuous delivery). Cold steel resection was performed using a number 10 blade scalpel.
Length measurement
Following resection, the specimen was laid flat on a microscope slide. The length of the resected specimens, resected either with cold steel or laser resection, was measured immediately in order to prevent the specimen drying and changing size.
All measurements were recorded by the same person in the dissection laboratory with digital callipers, to the nearest 0.01 mm, using the microscope for visualisation. There was no blinding to the resection technique during measurement.
All measurements were taken three times, and the average measurement recorded. For specimens with permission for photography, a photograph was taken using a tripod-mounted camera, with a scale placed below the microscope slide. The size of the specimen was measured again from the photograph using ImageJ software (Wayne Rasband, previously National Institutes of Health). The specimen was then fixed to cucumber using Loctite® Super Glue, and the length of the specimen was measured again, both with digital callipers and ImageJ software if permission for photography was available. Finally, the defect sizes in both the true and false vocal folds were measured using the digital callipers, while visualising the larynx through the microscope.
Data analysis and sample size calculation
A sample size calculation was performed, which indicated the need for a group size of at least three specimens per group. Data were recorded in Microsoft Excel spreadsheet software (Microsoft, Redmond, Washington, USA). Statistical analysis was performed in GraphPad Prism 8 software (GraphPad Software, San Diego, California, USA). The specimen size and defect size measurements were all assumed to be normally distributed. Descriptive statistics were performed on each dataset. The paired samples t-test was used to compare tissue shrinkage: between sites (true and false vocal fold), between resection methods (cold steel and laser), before and after application to cucumber, between defect sizes at different sites (true and false vocal fold), and between defect sizes depending on resection method (cold steel and laser). A p-value of less than 0.05 was taken to represent a statistically significant result.
Ethics and consent
The project was granted ethical approval from the Keele University School of Medicine Student Project Ethics Committee. The dissection laboratory operates under the license from the Human Tissue Authority. All cadaveric material had consent for use in teaching and research.
Results
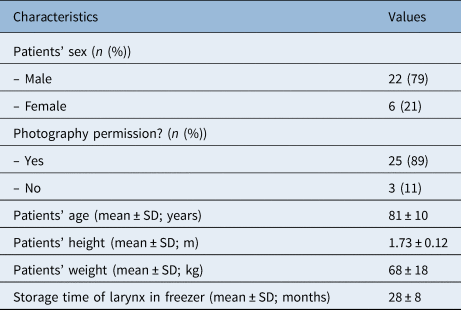
A total of 28 larynxes were made available for this study. The basic characteristics and descriptive statistics are displayed in Table 1. Twenty-five specimens had permission for photography and were able to have size measurements recorded using ImageJ software.
Table 1. Characteristics of patients and larynxes included in study
SD = standard deviation
There was shrinkage of all resected specimens, regardless of the location (true or false vocal fold), resection type (cold steel or laser) or measurement technique (digital callipers or ImageJ software) (Table 2, Figures 2 and 3). For cold steel (control) resection, shrinkage varied between 8 and 14 per cent; for laser resection, shrinkage varied between 35 and 45 per cent. The greatest shrinkage was noted for specimens from the false vocal fold resected with the laser, mounted on cucumber and measured with ImageJ software. In this group, the average specimen size measured 5.49 mm, a shrinkage of 45.06 per cent from the original 10 mm marked lesion.
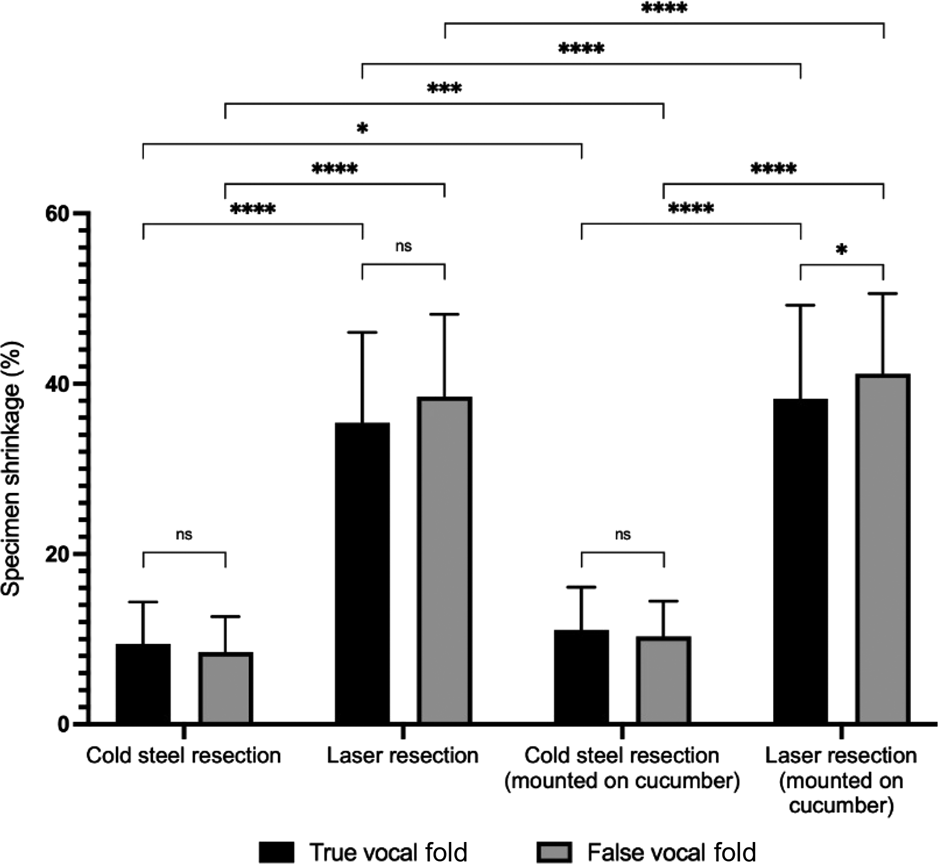
Fig. 2. Specimen shrinkage measured with digital callipers (n = 28), with paired comparisons. ns = non-significant; *p < 0.05; ***p < 0.001; ****p < 0.0001
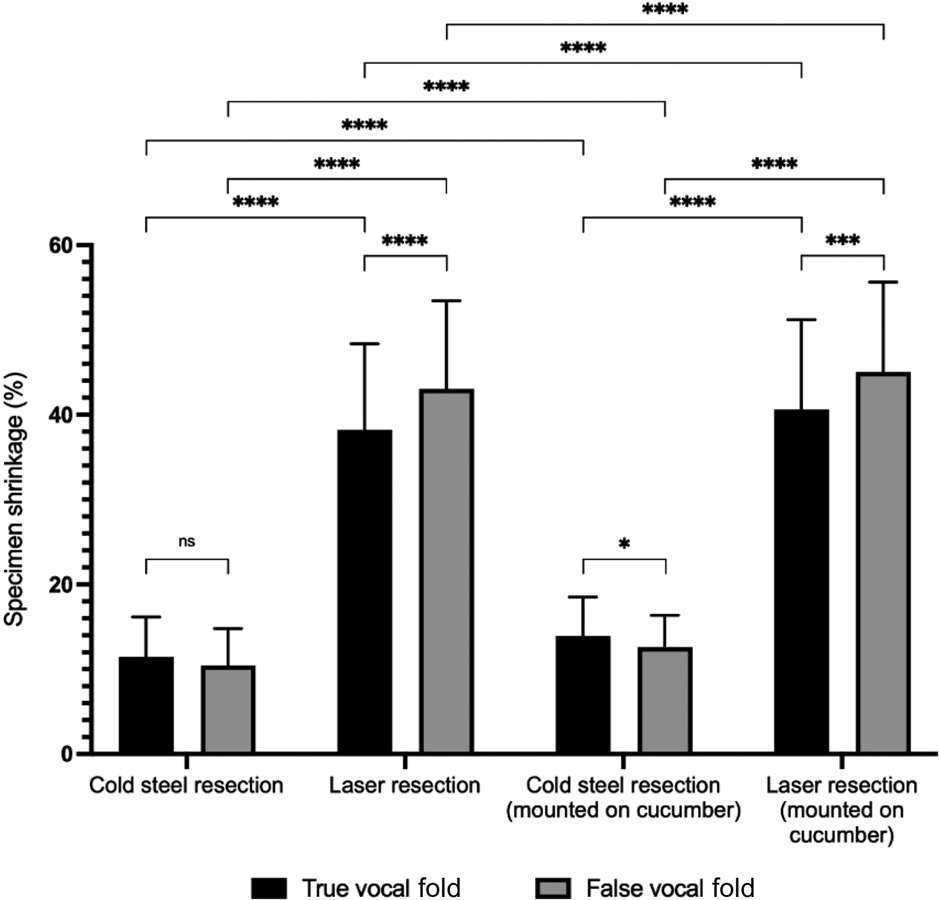
Fig. 3. Specimen shrinkage measured with ImageJ software (n = 25), with paired comparisons. ns = non-significant; *p < 0.05; ***p < 0.001; ****p < 0.0001
Table 2. Size and shrinkage of specimens at true and false vocal fold sites resected using cold steel and laser
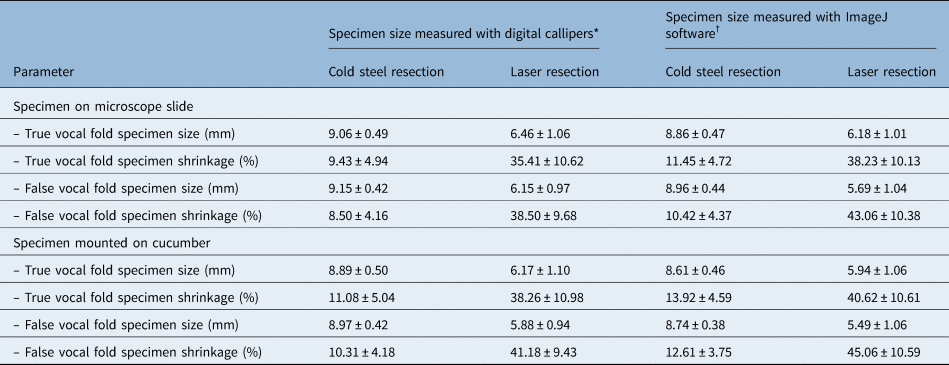
Data represent means ± standard deviations. *n = 28; †n = 25
Tissue shrinkage was significantly higher in specimens resected by laser, compared to those resected by the cold steel method (p < 0.0001) (Figures 2 and 3). For example, when measured with digital callipers at the true vocal fold without cucumber, shrinkage increased from 9.43 per cent with cold steel to 35.41 per cent with laser resection. Tissue shrinkage with laser resection was significantly higher than with cold steel resection in all cases, irrespective of the location of the specimen (true or false vocal fold), whether or not the specimen was mounted on cucumber, or whether the size was recorded with digital callipers or ImageJ software.
Mounting the specimen onto cucumber significantly reduced the specimen size (p < 0.0001 for most scenarios) (Figures 2 and 3). The p-value was higher (although still less than 0.05) for cold steel resections measured with digital callipers at both the true and false vocal fold sites.
Comparisons were made for tissue shrinkage between the true and false vocal fold sites. For cold steel dissection, average tissue shrinkage was greater at the true vocal fold compared to false vocal fold. Conversely, with laser resection, tissue shrinkage was greater at the false vocal fold compared to true vocal fold. These differences were not all statistically significant.
The defect sizes in the larynx following resection of the specimen were all greater than 10 mm, ranging from an average of 10.37 mm in the true vocal fold cold steel resection (smallest average defect) to 11.27 mm in the true fold laser resection (largest average defect) (Table 3 and Figure 4). There was no significant difference with either cold steel or laser resection when comparing the difference in defect sizes between the true and false fold specimens. Laser resection resulted in a larger defect compared to cold steel resection; this was significantly larger at the true vocal fold site (11.27 mm compared to 10.37 mm, p = 0.0002).
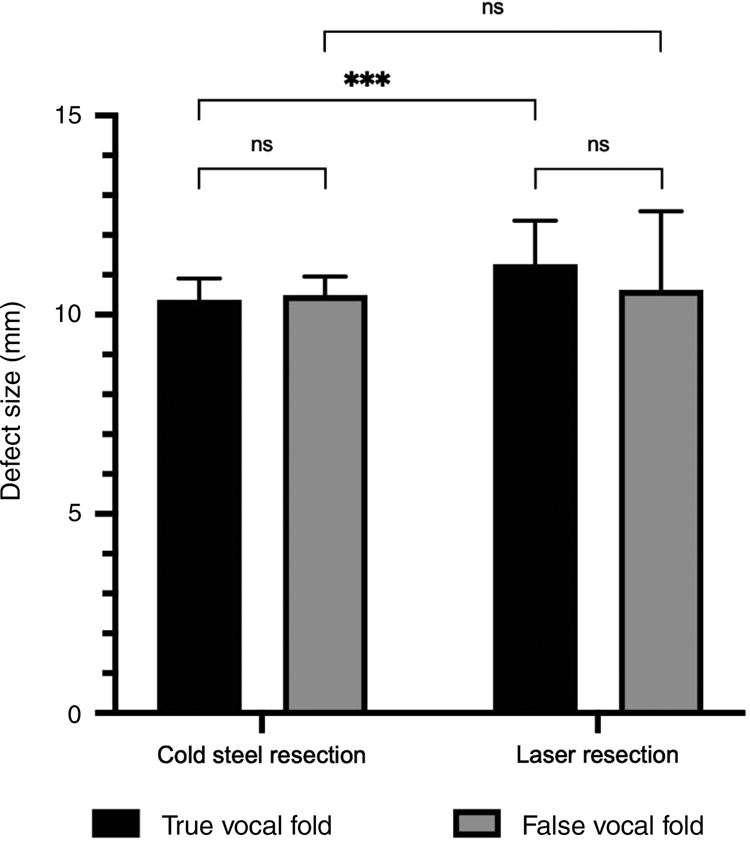
Fig. 4. Defect sizes measured after excision of the laryngeal specimens (n = 28), with paired comparisons. ns = non-significant; ***p < 0.001
Table 3. Defect sizes in true and false vocal fold specimens resected using cold steel and laser

Data represent means ± standard deviations.
Discussion
In oncological surgery, obtaining clear surgical margins that are free from cancer is crucial. An unsatisfactory surgical margin is an independent risk factor for recurrence-free survival as well as overall survival.Reference Eldeeb, Macmillan, Elwell and Hammod14 The head and neck surgeon may find that the margin reported by the histopathologist is significantly less than the anticipated margin from the surgical resection. This discrepancy is due to shrinkage of the resected specimen, which can affect the size of the margin. Additionally, the effect of thermalisation from the CO2 laser may further hinder accurate reporting of the surgical margin, compounding the effect of tissue shrinkage.
A study from 1986 reported on the shrinkage of the oesophagus after resection for carcinoma.Reference Siu, Cheung and Wong15 The upper and lower margins of the specimen were respectively reduced to 44 per cent and 54 per cent of their in situ lengths. This provided early documentation for shrinkage of surgical specimens, which may explain the discrepancy claimed by surgeons and pathologists regarding margin length.
In 1991, a melanoma research group in New York studied the shrinkage of cutaneous surgical specimens from 199 malignant melanomas.Reference Golomb, Doyle, Grin, Kopf, Silverman and Levenstein16 They were able to devise a formula to calculate the in vivo (pre-excision) specimen diameter from the in vitro (fixed-tissue) specimen diameter. This research group validated their findings in 1992.Reference Silverman, Golomb, Kopf, Grin-Jorgensen, Vossaert and Doyle17 A total of 407 patients with malignant melanoma were included in the prospective study that measured pre-excision surgical margins and compared them with fixed-tissue (contracted) margins. The overall shrinkage of specimens was around 20 per cent. They found that the shrinkage was higher in younger patients (25 per cent in patients aged less than 50 years, 20 per cent in patients aged 50–59 years, and 15 per cent in patients aged 60 years and older).
In head and neck surgery, using a canine model, one group attempted to quantify the change in size of the mucosal and muscle surgical margins following excision, formalin fixation, and slide preparation of tongue and labiobuccal tissue.Reference Johnson, Sigman, Funk, Robinson and Hoffman18 In that study, published in 1997, 10 mongrel dogs underwent excision around custom-made discs, and the excised specimens were measured immediately after excision, after formalin fixation and after slide preparation. The mean shrinkage from initial resection to final microscopic assessment was 30.7 per cent at the lingual surface mucosal margins, 34.5 per cent at the deep tongue margin and 47.3 per cent at the labiobuccal mucosal margin. In that study, in order to obtain a 5 mm pathologically clear margin at histological assessment, the surgical margin needed to be at least 8–10 mm.
In a human study of 27 patients with carcinoma of the tongue and buccal mucosa, mucosal margins were assessed prior to resection and half an hour after excision.Reference Mistry, Qureshi and Kumaran11 The mean shrinkage of the tongue margins was 23.5 per cent, compared to 21.2 per cent for buccal mucosal margins. This paper found that the shrinkage for stage T1 and T2 tumours (25.6 per cent) was significantly greater compared to T3 and T4 tumours (9.2 per cent). The study suggested that shrinkage should be considered, to prevent concerns with positive post-operative margins when assessed by the histopathologist.
Another prospective study, published in 2014, investigated 10 larynxes excised from patients with advanced laryngeal cancer who had undergone laryngectomy, with a view to assessing the thermal effect caused by surgical incisions using a scalpel, CO2 laser, harmonic scalpel and electrocautery.Reference Mannelli, Meccariello, Deganello, Maio, Massi and Gallo19 Incisions were made on macroscopically healthy laryngeal mucosa, some distance from the primary tumour. Using the scalpel incision as a control, the study quantified the amount of shrinkage for each of the other three resection techniques. The CO2 laser resulted in the largest amount of shrinkage, with a mean shrinkage of 2.91 mm, compared to the surgical margin with the scalpel. The site of the incisions was not reported as being consistent between specimens, and it was not possible to determine the amount of shrinkage, which may also have been the result of scalpel incision. The larynx is divided into three regions (supraglottis, glottis and subglottis) with different histology and structures; it is possible that the shrinkage profile may be different between regions. It was not clear that the study allowed for this, as the excisions were not consistently taken from the same locations on each larynx.
One study investigated the effect of formalin fixation on specimen size. In 100 head and neck cancer specimens excised during surgery, the specimens were measured immediately after resection and again after formalin fixation.Reference Chen, Hsu, Jiang, Wu, Chen and Liu20 The specimens decreased in size by 4–6 per cent after fixation. The authors commented that care must be taken when interpreting specimen sizes so as to not underestimate the true size of the tumour.
Transoral laser microsurgery using the CO2 laser is a popular technique for the management of early-stage laryngeal cancer. The current study has quantified the shrinkage of laryngeal specimens taken from both the true and false vocal folds, comparing laser resection to cold steel resection. We have tried to minimise discrepancy in size measurements by measuring all specimens three times with digital callipers and recording the average value, while viewing the specimen through the operating microscope to improve accuracy. For those specimens with permission for photography, the measurements were taken a second time using digital analysis, to reduce subjective changes in measurement technique. All specimens were taken from the same location on each specimen, and both supraglottic (false vocal fold) and glottic (true vocal fold) tissue were analysed to account for any possible differences in shrinkage profiles for different areas of the larynx.
• Transoral laser microsurgery using carbon dioxide laser is a treatment option in early-stage laryngeal cancer
• Heat from the laser can cause tissue shrinkage of the specimen and affect the surgical margin
• Previous studies have demonstrated shrinkage of oesophagus, skin, and head and neck tissue specimens after excision
• This study quantifies the amount of shrinkage of laryngeal specimens taken from true and false vocal folds, comparing laser with cold steel resection
• The results have implications for surgical practice; awareness of shrinkage is important when considering close margins, in terms of planning further treatment
Our results demonstrate shrinkage of all specimens, regardless of their original location (true or false vocal fold), resection technique (cold steel or laser) or mounting technique (with or without cucumber). Laser resection resulted in average shrinkage of between 35 and 45 per cent, which was significantly more than tissue shrinkage with cold steel resection (8–14 per cent). Mounting the specimens on cucumber also significantly increased shrinkage.
The present study was performed on fresh-frozen larynxes. The effect of freezing and rewarming may affect the water content of tissues compared to in vivo samples. We hypothesise that the frozen larynx will hold more water than in vivo samples. This is likely to result in a reduced thermalisation effect from the laser, possibly leading to an underestimate of the degree of tissue shrinkage in our study.
The results have implications for surgical practice. Assuming average shrinkage of 40 per cent for laser resection, a 1 mm margin recorded at the time of surgery will measure only 0.6 mm when reported by the histopathologist, and will be described as a close margin, potentially prompting discussions about further treatment. Awareness of this shrinkage is important to help inform management plans. Conversely, the surgeon would need to remove a specimen using the laser with a margin of 1.7 mm in order to allow for a 1 mm margin as measured by the pathologist.
Conclusion
Early-stage laryngeal cancer can be treated with transoral laser microsurgery using the CO2 laser. The human larynx is a relatively small anatomical structure, and excess removal of normal, healthy tissue during cancer surgery should be minimised. This study demonstrates that tissue specimens from the larynx shrink after excision. Laser resection results in significantly more tissue shrinkage compared to cold steel resection. Surgeons and pathologists should be aware of this shrinkage when considering positive and close margins, to ensure that future treatment plans for the patient are appropriate.
Competing interests
None declared









