Introduction
Acute rhinosinusitis is predominantly caused by upper airway viral infections which are self-limiting; management comprises symptomatic relief and patient reassurance.Reference Fokkens, Lund, Mullol and Bachert1 The routine use of antimicrobial therapy is not indicated for this disease. However, a small number of patients develop secondary acute bacterial rhinosinusitis, and their management necessitates the use of antimicrobial drugs.Reference Fokkens, Lund, Mullol and Bachert1 An accurate diagnosis depends on a thorough clinical assessment of patients, as commonly performed by general practitioners in the UK. Acute bacterial rhinosinusitis is generally treated in the community in various primary care settings. National bodies and local hospitals have produced guidelines in the form of algorithms to improve the diagnosis of this disease and optimise the use of antimicrobial drugs.
Demonstration of a bacterial infection in the paranasal sinuses is the ‘gold standard’ for diagnosing acute bacterial rhinosinusitis. Paranasal sinus pus obtained via sinus washout is a much better clinical sample compared with a nasal swab. Bacterial culture from nasal or throat swabs does not accurately reflect clinical disease because it is very difficult to distinguish commensal from pathogenic bacteria. Unfortunately, sinus washout is not routinely performed in a clinical or primary care setting. Imaging is also used for acute rhinosinusitis diagnosis but cannot differentiate between viral and bacterial infections.Reference Gwaltney, Phillips, Miller and Riker2 Therefore, a combination of clinical assessment, imaging and microbiological analysis is considered the optimum method of diagnosing acute bacterial rhinosinusitis. However, this approach is not possible in primary care settings and may not even be available in hospital settings. Therefore, empirical antimicrobial therapy is often based on local guidelines and experience.
The Infectious Diseases Society of America (‘ISDA’) recently published guidelines for treating acute bacterial rhinosinusitis.Reference Chow, Benninger, Brook, Brozek, Goldstein and Hicks3 These guidelines highlighted the difficulty of microbiological sampling and the limitations of microbiology findings reported in many clinical trials of sinusitis treatment. They recommend the use of broad-spectrum antimicrobial drugs, such as co-amoxiclav, as first-line therapy, partly in response to reports of an increased resistance of Streptococcus pneumoniae to penicillins.Reference Brook4
There is a current lack of knowledge about the microbiology of complicated acute bacterial rhinosinusitis. This study therefore aimed to review our empirical treatment regimens based on more accurate microbiological analyses. A retrospective evaluation of recent complicated acute bacterial rhinosinusitis cases from our institute was conducted. The principal aim was to describe the bacterial pathogens and their susceptibility to antimicrobial drugs commonly used in this setting along with any corresponding sinogenic complications such as an orbital or intracranial abscess. A secondary aim was to extrapolate the results to provide guidance on treating uncomplicated acute bacterial rhinosinusitis in the community.
Materials and methods
Study design and patient selection
This retrospective study evaluated complicated acute bacterial rhinosinusitis patients referred to the Leeds Teaching Hospitals NHS Trust (UK) during the period from 1 January 2007 to 31 December 2012. Complicated acute bacterial rhinosinusitis was defined as acute bacterial rhinosinusitis plus orbital and/or intracranial infection.
Inclusion criteria were those previously defined by Manning et al.Reference Manning, Biavati and Phillips5 These comprised documented clinical evidence of acute bacterial rhinosinusitis, as suggested by the presence of at least three of the following: symptoms and/or signs: discoloured nasal discharge (with unilateral predominance), severe local pain (with unilateral predominance), fever (over 38 °C) and ‘double sickening’ (i.e. deterioration after an initial milder phase). Additional inclusion criteria were bacterial culture from a surgically obtained paranasal sinus pus sample with or without orbital and/or intracranial abscess pus samples, and computed tomography (CT) evidence of acute bacterial rhinosinusitis with or without an orbital and/or intracranial abscess.
Patients for whom nasal swabs only, swabs taken during elective surgery for chronic rhinosinusitis or swabs from non-paranasal sinuses were available, and those with post-traumatic sinusitis or without CT scans were excluded.
Data collection
Firstly, a database of all patients with paranasal sinus pus and orbital and intracranial abscess samples positive for bacterial infection between January 2007 and December 2012 was established. Data were retrospectively collected from the medical notes and from hospital electronic and radiology databases, results were stored using Microsoft Office Excel 2007 (Microsoft, Redmond, Washington, USA). Pre-admission antibiotic therapy was determined from case notes, the electronic discharge system and general practitioner referral letters, where traceable. All CT images were reviewed and compared with reports provided by radiologists. A database of bacteria cultured from paranasal sinus pus and orbital, frontal sinus and intracranial abscesses was cross-referenced with the documented clinical features and CT imaging data. This process enabled complicated acute bacterial rhinosinusitis to be accurately identified.
Antimicrobial therapy
After hospital admission, all complicated acute bacterial rhinosinusitis patients with orbital and/or intracranial infection were initially treated empirically with intravenous cefotaxime, metronidazole and flucloxacillin. The antibiotic regimen was subsequently altered based on the patient's response and microbiology results and microorganism susceptibility. Treatment duration was guided by the extent of infection, the clinical response, radiological monitoring and the type of microorganism(s) isolated.
Microbiological specimens
Paranasal sinus pus samples were obtained during surgery using an endoscopic approach from the maxillary or ethmoid sinuses and via the frontal sinus trephine. Pus samples and swabs were obtained from orbital abscess drainage via an external approach and from intracranial abscesses via a burr hole or craniotomy, as appropriate. All specimens were promptly sent for microbiological analysis comprising microscopy, bacterial culture and antibiotic susceptibility assessment.
Microbiological analysis
For bacterial culture, wound swabs were incubated at 37 °C for two days on horse blood agar in 5–10 per cent carbon dioxide (CO2), on cysteine lysine electrolyte deficient ager in air and on neomycin horse blood agar under anaerobic conditions. Pus samples were incubated at 37 °C on horse blood agar and chocolate agar in 5–10 per cent CO2 for two days, on cysteine lactose electrolyte deficient ager in air for two days, on neomycin horse blood agar under anaerobic conditions for three days and on fastidious anaerobe broth for one day. Tissue specimens were incubated at 37 °C on horse blood agar and chocolate agar in 5–10 per cent CO2 for three days, on fastidious anaerobe agar under anaerobic conditions for five days, on Sabouraud agar in air for seven days and in brain–heart infusion broth for five days. Anaerobic conditions were achieved using a MACS-MG-1000 anaerobic workstation (DW Scientific, Shipley, UK). Bacteria were identified using standard techniques and antibiotic susceptibility testing was performed using a disc diffusion method according to European Committee on Antimicrobial Susceptibility Testing (‘EUCAST’) criteria. Molecular biology techniques were not used for sample analysis.
Ethical considerations
Formal study approval was obtained from the local clinical department. Ethical approval was not necessary because the project was categorised as a service evaluation and did not affect patient care at any level. Data were recorded and stored in accordance with Caldicott Guardianship protocols.
Results
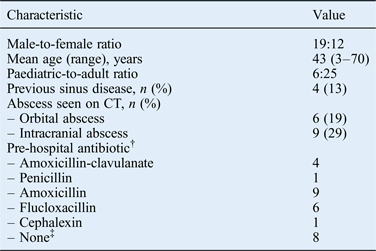
After removing duplicate cases generated by the patient identification strategy (n = 13), a total of 31 patients met the strict inclusion criteria for the study (Figure 1). Patient demographic details are shown in Table I. There were 29 cases of primary acute bacterial rhinosinusitis and 2 cases secondary to odontogenic pathology. These included 16 cases of acute bacterial rhinosinusitis plus periorbital cellulitis, 15 of acute bacterial rhinosinusitis with suspected intracranial abscess on imaging criteria, and 9 intracranial and 6 orbital abscesses (Table I). There was a history of sinus disease in four patients (12.9 per cent). Twenty-one (67 per cent) patients had taken oral antibiotics prior to hospital admission; five of these had taken broad-spectrum antibiotics.

Fig. 1 Flowchart of patient selection for inclusion in the study.
Table I Patient demographics and clinical characteristics*

*n = 31. †For two patients, data on pre-hospital antibiotic treatment could not be traced due to a lack of documentation. ‡Patients presented directly to the hospital emergency department. CT = computed tomography
Streptococcus anginosus group bacteria were isolated from paranasal sinus pus samples from 61.3 per cent of patients (19 out of 31), including 83.3 per cent of orbital abscesses (5 out of 6) and 77.8 per cent of intracranial abscesses (7 out of 9; Table II). For all orbital and intracranial abscesses in which S anginosus was identified, S anginosus cultures were obtained from the corresponding paranasal sinus pus samples. Beta-haemolytic streptococci were the next commonest streptococcal organisms isolated from paranasal sinus pus samples (16.1 per cent). However, these bacteria were not isolated from orbital or intracranial abscesses.
Table II Micoorganisms most commonly isolated from culture specimens

Staphylococcus aureus was the second commonest pathogen; it was isolated from 12 paranasal sinus pus samples (38.7 per cent). S aureus was always isolated with another organism, most notably a Streptococcus spp. In particular, S aureus was identified in 66 per cent of orbital abscesses and 44 per cent of intracranial abscesses.
Multiple organisms (S anginosus plus other organisms) were isolated in 41.9 per cent of paranasal sinus pus samples. Stenotrophomonas maltophilia was isolated from a single paranasal sinus pus sample. This patient was immunocompromised but did not develop an orbital or intracranial abscess (periorbital cellulitis only). S anginosus and Gram-negative anaerobic bacteria were isolated from one orbital abscess sample and one intracranial abscess sample. Haemophilus influenzae was isolated from one orbital abscess sample.
All S anginosus cultures (whether isolated from paranasal sinus or abscess samples) and all beta-haemolytic streptococci were susceptible to penicillin. The H influenzae isolate was also susceptible to amoxicillin. S aureus isolates were resistant to penicillin but susceptible to methicillin (flucloxacillin).
Discussion
The bacterial species responsible for acute bacterial rhinosinusitis are similar to those found in community-acquired pneumonia: S pneumoniae, H influenzae, Moraxella catarrhalis and, occasionally, S aureus.Reference Chow, Benninger, Brook, Brozek, Goldstein and Hicks3 Acute bacterial rhinosinusitis generally follows a viral respiratory tract infection and can be secondary to odontogenic infection, surgery or trauma. Most patients can be managed with oral antimicrobial drugs in a primary care setting. Only a small minority may require hospital care because of a failure to resolve or the development of complicated acute bacterial rhinosinusitis. The microbiology of complicated acute bacterial rhinosinusitis is not well described (generally out of date and country specific) but does appear to differ from that of uncomplicated acute bacterial rhinosinusitis.Reference Mortimore, Wormald and Oliver6
In this disease evaluation in a UK population, S anginosus group bacteria were the predominant cause of acute bacterial rhinosinusitis (61 per cent) and of the corresponding orbital (83 per cent) and/or intracranial abscesses (78 per cent). In all cases, these organisms were susceptible to penicillin. Interestingly, this S anginosus incidence is much higher than previously reported. In 1998, Mortimore et al. described the microbiology of complicated sinusitis in a developing country, and reported S anginosus isolates in 50 per cent of abscesses.Reference Mortimore, Wormald and Oliver6 In 1981, Gwaltney et al. reported that S aureus was the second commonest pathogen (38 per cent) in acute sinusitis and that methicillin-resistant strains were not identified.Reference Gwaltney, Sydnor and Sande7 A substantial proportion of patients (42 per cent) had a mixed infection. S pneumoniae, H influenzae and M catarrhalis were uncommon in acute bacterial rhinosinusitis patients. The microbiology of complicated and uncomplicated disease appears to be markedly different.
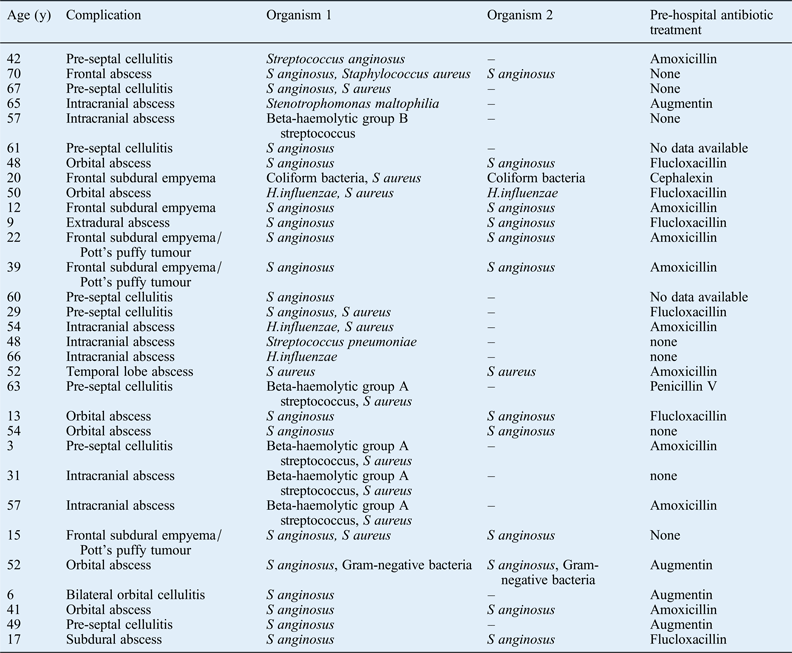
Antibiotic treatment prior to hospital admission with an acute bacterial rhinosinusitis complication does not appear to have any impact on the complication type (Table III). In all, 21 patients received a pre-hospital course of oral antibiotics. The reason that acute bacterial rhinosinusitis patients develop a sinogenic complication despite a course of oral antibiotics may relate to the causative microorganism: S aureus and S anginosus are well-known causes of abscesses and these pathogens may have a greater risk of complications. However, the reason that some acute bacterial rhinosinusitis patients develop complications is likely to be multifactorial, involving their smoking history, their genetic background, and host immune system functions including anatomical barriers and biofilms.Reference Brook and Hausfeld8 However, a detailed discussion of these factors is beyond the scope of this article. It is noteworthy that the micro-organisms isolated from eight patients who presented directly to the emergency department, and thus had no prior antibiotic treatment, were not markedly different from those of patients presenting with prior antibiotic treatment.
Table III Pre-hospital antibiotic therapy by patient and complication

y = years
Although the organisms responsible for acute bacterial rhinosinusitis are assumed to occasionally lead to sinogenic complications necessitating admission to hospital, this has rarely been described.Reference Mortimore, Wormald and Oliver6, Reference Brook and Frazier9, Reference Brook10 However, the present study showed a 100 per cent agreement in bacterial species isolated from paranasal sinus and abscess samples.
It is important to recognise the specific features suggestive of acute bacterial, rather than viral, acute rhinosinusitis (the latter constitutes approximately 90 per cent of cases). The following features are suggestive of acute bacterial rhinosinusitis: (1) onset with persistent symptoms or signs compatible with acute rhinosinusitis, lasting for 10 days without any evidence of clinical improvement; (2) onset with severe symptoms or signs of high fever (39 °C) and purulent nasal discharge or facial pain, lasting for at least 3–4 consecutive days at the beginning of illness; or (3) onset with worsening symptoms or signs characterised by a new onset of fever or headache or by increased nasal discharge following a typical viral upper respiratory infection lasting 5–6 days that initially improves (‘double sickening’).Reference Chow, Benninger, Brook, Brozek, Goldstein and Hicks3 Although the triad of headache, facial pain and fever is considered a classic presentation of acute bacterial rhinosinusitis in adults, it is uncommon: onset with persistent symptoms is far more common.Reference Fokkens, Lund, Mullol and Bachert1 In children, the most common manifestations of acute bacterial rhinosinusitis are coughing followed by nasal discharge and fever.
In recent years, several studies have reported that S pneumoniae is the microorganism most often responsible for acute bacterial rhinosinusitis that is highly resistant to first-line antibiotics in the USA.Reference Brook4 Methicillin-resistant S aureus has also been reported in both complicated and uncomplicated acute bacterial rhinosinusitis, with a prevalence of 0–15.9 per cent.Reference McCoul, Jourdy, Schaberg and Anand11 These phenomena both contradict the findings of the current study in a UK population: S anginosus and methicillin-sensitive S aureus were the most common pathogens in complicated acute bacterial rhinosinusitis and there were no resistant organisms. In an era of increasing antibiotic resistance, this is a very important finding that highlights the importance of obtaining local and national data on the antibiotic sensitivity of pathogens before implementing international guidelines (such as those proposed by the Infectious Diseases Society of America) for treating acute bacterial rhinosinusitis.
This study also shows that for immunosuppressed patients broad-spectrum antibiotics and ENT consultation may need to be considered from the outset because of the possibility of unusual organisms which may not be sensitive to first-line antibiotics, as recommended by most guidelines. It is also notable that Gram-negative organisms were also isolated from both patients presenting with sinogenic complication secondary to dental infections.
• There was 100 per cent agreement between the microbiology profiles of direct sinus and abscess samples
• Streptococcus anginosus was the bacterium most frequently isolated from paranasal sinus, orbital abscess and intracranial abscess samples
• S anginosus is sensitive to penicillin
• Therefore, penicillin is an appropriate first-line empiric antibiotic therapy for uncomplicated acute bacterial rhinosinusitis
Study limitations
This was a retrospective study and as such may be limited by the methodology used. It examined only complicated acute bacterial rhinosinusitis to obtain disease-specific microbiological cultures, which are not usually available with uncomplicated rhinosinusitis. However, the results have important implications for the antibiotic treatment of acute rhinosinusitis in an era of increasing antibiotic resistance. The sample size was small and, considering the low overall incidence of complicated acute bacterial rhinosinusitis, it is reasonable to interpret the study findings with a degree of caution. It is important to note that the high bacterial culture rates in this study were achieved because invasive sampling at a specific site was an inclusion criterion. The study data has been used as a basis for recommendations for treating uncomplicated acute bacterial rhinosinusitis in the community. However, a degree of caution should be exercised when treating patients in regions outside the UK.
Conclusion
This study describes the current causative micro-organisms for acute bacterial rhinosinusitis in a UK population. The Infectious Diseases Society of America Clinical Practice Guideline for Acute Bacterial Rhinosinusitis in Children and Adults (2012) recommends using co-amoxiclav rather than amoxicillin alone as the initial empirical therapy.Reference Chow, Benninger, Brook, Brozek, Goldstein and Hicks3 The microorganism most often involved in complicated acute bacterial rhinosinusitis in this study was S anginosus, which is not resistant to simple penicillins such as amoxicillin and penicillin V. S aureus was an important pathogen in patients with complicated disease. There do not appear to be the same epidemiological changes in the UK as in the USA to justify more broad-spectrum empirical regimens. However, in complicated disease, empirical treatment for streptococci and S aureus appears to be appropriate. We recommend the use of simple narrow-spectrum penicillins as first-line antibiotic therapy for uncomplicated acute bacterial rhinosinusitis in the community, and do not advocate changing the current guidelines for treating this disease. In the management of complicated acute bacterial rhinosinusitis, however, we recommend using an antibiotic regimen effective against both S anginosus and S aureus.