Differentiated thyroid cancer
Introduction
Thyroid nodules are common, the incidence of palpable nodules in women and men being approximately 5 and 1 per cent, respectively. Use of ultrasound scanning (USS) substantially increases their detection in the general population to approximately 50–70 per cent. Thyroid cancer remains rare, with an incidence in the UK of approximately 5 per 100 000 women and 2 per 100 000 men. Thyroid cancer is the most common endocrine malignancy, but accounts for only 1 per cent of all malignancies. Evidence suggests an increasing incidence; however, the survival rates remain static.
Long-term prognosis for differentiated thyroid cancer (DTC) is excellent, with survival rates for adults being 92–98 per cent at 10-year follow-up. However, 5–20 per cent of patients develop local or regional recurrence requiring further treatment and 10–15 per cent go on to develop distant metastases. Factors influencing prognosis include gender, age at presentation, histology and tumour stage. Accurate diagnosis, treatment and long-term follow-up are essential to achieve and maintain excellent survival rates.
There have been several sets of detailed guidelines published on the diagnosis and management of thyroid cancer. Two key ones are the Guidelines for the Management of Thyroid Cancer (2014) by the British Thyroid Association and Royal College of Physicians,Reference Perros, Boelaert, Colley, Evans, Evans and Gerrard Ba 1 and the Revised American Thyroid Association Guidelines (2016).Reference Haugen, Alexander, Bible, Doherty, Mandel and Nikiforov 2 These documents are extensive and cover every aspect of care in great detail. Given differences in presentation, pathophysiology and outcomes, separate guidelines exist for children with DTC,Reference Francis, Waguespack, Bauer, Angelos, Benvenga and Cerutti 3 and consensus statements on the various surgical interventions.Reference Lorenz, Niederle, Steinmuller and Dralle 4 Patients may initially be seen by a surgeon, endocrinologist, clinical oncologist or nuclear medicine physician, who must be a core member of the thyroid cancer multidisciplinary team (MDT). The goals of treatment for DTC are set out in Box I.
BOX I GOALS OF TREATMENT FOR DTC
-
• Remove the primary tumour and involved lymph nodes
-
• Minimise treatment related morbidity
-
• Allow accurate staging of the disease
-
• Facilitate post-operative treatment with radioactive iodine in appropriate patients
-
• Enable long-term surveillance for disease recurrence
-
• Minimise the risk of disease recurrence and distant metastases
Clinical presentation
In all cases, a detailed history is required. Clinical features associated with an increased risk of malignancy in individuals with a thyroid nodule include:
-
• age younger than 20 or older than 60 years
-
• firmness of the nodule on palpation
-
• rapid growth
-
• fixation to adjacent structures
-
• vocal cord paralysis
-
• associated lymphadenopathy
-
• history of neck irradiation
-
• family history of thyroid cancer
-
• history of Hashimoto's thyroiditis (risk factor for thyroid lymphoma).
Symptoms warranting immediate referral
Patients presenting with airway compromise, including stridor, associated with a thyroid nodule or goitre should be referred for an immediate opinion.
Symptoms warranting urgent general practitioner (GP) referral (two-week wait rule)
Patients presenting with hoarseness of voice or a change in their voice associated with a thyroid nodule or goitre, children with a thyroid nodule, individuals with cervical lymphadenopathy associated with a thyroid nodule or a painless thyroid mass, which is rapidly enlarging over a period of weeks should be referred for an urgent opinion.
Investigation
Recommended clinical investigations
These include:
-
• Clinical evaluation of thyroid, cervical and supraclavicular nodes
-
• Thyroid-stimulating hormone (TSH)
-
• Ultrasound of the nodule (Table I)
-
• Fine needle aspiration cytology (FNAC) if ultrasound features are suspicious of malignancy
-
• Documented cytological score (Table II). A core biopsy (with or without USS guidance) is warranted if a diagnosis of lymphoma is suspected
-
• Calcitonin only in suspected cases of medullary thyroid cancer (MTC) (routine use not recommended)
-
• Pre-operative vocal cord check
-
• Note that a serum thyroglobulin (Tg) is not recommended.
Table I U grading of thyroid nodules

FNAC = fine needle aspiration cytology
Table II Thyroid FNAC diagnostic categories

* Hemithyroidectomy consists of removal of a thyroid lobe and the isthmus
Ultrasound of thyroid nodules
Ultrasound is very useful in the investigation of thyroid nodules and should be used to guide the need for further investigation including FNAC. Ultrasound-guided FNAC increases the yield of diagnostic cytology significantly. Current guidelines recommend that ultrasonographers use the U grade (Table I) to classify nodules according to ultrasound appearances.Reference Leenhardt, Erdogan, Hegedus, Mandel, Paschke and Rago 5
Ultrasound evaluation of cervical lymphadenopathy
Pathological studies suggest that microscopic lymph node metastases are very common in papillary thyroid cancer (PTC). However, macroscopic disease is less common (20–50 per cent). Pre-operative ultrasonography is effective in identifying suspicious nodes in approximately 20–30 per cent of patients with PTC and may alter the surgical approach. FNAC of suspicious nodes is recommended. Tg estimation of cystic fluid may be of use in the absence of sufficient diagnostic material.
Recommendations
-
• Ultrasound scanning of the nodule or goitre is a crucial investigation in guiding the need for FNAC (R)
-
• FNAC should be considered for all nodules with suspicious ultrasound features (U3–U5). If a nodule is smaller than 10 mm in diameter, USS-guided FNAC is not recommended unless clinically suspicious lymph nodes on USS are also present (R)
-
• Cytological analysis and categorisation should be reported according to the current British Thyroid Association Guidance (R)
-
• Ultrasound scanning assessment of cervical nodes should be done in FNAC-proven cancer (R)
-
• Magnetic resonance imaging (MRI) or computed tomography (CT) should be done in suspected cases of retrosternal extension, fixed tumours (local invasion with or without vocal cord paralysis) or when haemoptysis is reported. When CT with contrast is used pre-operatively, there should be a two-month delay between the use of iodinated contrast media and subsequent radioactive iodine therapy (R)
-
• Fluoro-deoxy-glucose-positron emission tomography imaging is not recommended for routine evaluation (G)
Staging
The tumour, nodes and metastases (TNM) staging system (Table III) is used to stage thyroid cancers and this should be used in all cases. Post-operatively, an ‘R’ classification can be given which indicates the amount of residual disease present. The TNM classification can then be used in combination with patient characteristics to define likely prognosis (Table IV).
Table III Tumour, nodes and metastases 7th edition staging system for differentiated thyroid cancer

MDT = multidisciplinary team
Table IV Group staging and survival for differentiated thyroid cancer

* Undifferentiated or anaplastic carcinomas are all stage IV
Surgery
Surgeons performing operations for confirmed or suspected thyroid cancer should be core members of the thyroid cancer MDT and should perform a minimum of 20 thyroidectomies per year. Complex surgery and lymph node surgery should be undertaken by nominated surgeons in the cancer centre with specific training in, and experience of, thyroid oncology. All patients with suspected or confirmed thyroid cancer should have pre-operative imaging with ultrasound. Cross-sectional imaging with CT or MRI may also be indicated.
In the context of thyroid cancer, surgery may be diagnostic (e.g. hemithyroidectomy following Thy 3 or Thy 4 cytology) or therapeutic.
Thyroid surgery for papillary thyroid cancer (PTC)
A strategy for the surgical treatment of PTC is detailed in Table V. All cases should be discussed pre-operatively at the thyroid cancer MDT.
Table V Initial surgery for papillary thyroid carcinoma

Initial surgery for follicular thyroid cancer
The majority of patients undergoing surgery for follicular thyroid cancer will be undiagnosed at the time of the initial surgery (Thy 3). Frozen section histology cannot currently reliably differentiate benign follicular lesions from follicular thyroid cancer, and therefore this strategy is not recommended. An operative strategy for surgical treatment of follicular cancer is outlined in Table VI.
Table VI Initial surgery for follicular thyroid cancer

Low-risk patients with a diagnosis of minimally invasive tumour less than 4 cm following hemithyroidectomy do not require further treatment. Hurthle cell cancers (follicular oncocytic) tend to be more aggressive and should be treated by total (completion) thyroidectomy (see Table VI).
Management of lymph nodes in differentiated thyroid cancer (DTC)
Prophylactic level VI lymph node dissection is associated with a higher incidence of recurrent laryngeal nerve damage and long-term permanent hypoparathyroidism.Reference Sancho, Lennard, Paunovic, Triponez and Sitges-Serra 6 It is therefore not routinely recommended, but in individuals with high-risk tumours, this should be discussed in the spirit of personalised decision making. Prophylactic level VI nodal dissection is not recommended in low risk, small papillary and most follicular cancers.
Prophylactic level VI nodal dissection is recommended in patients with known involved lateral nodes. Therapeutic level VI nodal dissection is recommended when the presence of lymph node metastasis is confirmed.
Clinically involved lateral cervical lymph nodes should be managed by selective neck dissection (levels II–V). Involvement of level I or VII node is rare in DTC and should only be dissected if involved. Prophylactic lateral neck compartment dissection for node negative patients is not recommended.
Completion thyroidectomy
Completion thyroidectomy is not needed in low-risk, unifocal, intrathyroidal tumours less than 4 cm in diameter, with clinically negative lymph nodes.
Locally advanced disease
Where possible, locally advanced disease should be resected. Preservation of recurrent laryngeal nerves should be attempted in almost all cases. Extensive resection of trachea, larynx and oesophagus should be considered if potentially curative. Where disease is unresectable, radiotherapy and radioiodine should be considered.
Microcarcinomas
Microcarcinomas are differentiated thyroid carcinomas less than 10 mm in maximum dimension and are predominantly papillary carcinomas. The management of papillary microcarcinomas is outlined in Figure 1.
Recommendations
-
• In patients with thyroid cancer, assessment of extrathyroidal extension and lymph node disease in the central and lateral neck compartments should be undertaken pre-operatively by USS and cross-sectional imaging (CT or MRI) if indicated (R)
-
• For patients with Thy 3f or Thy 4 FNAC a diagnostic hemithyroidectomy is recommended (R)
-
• Total thyroidectomy is recommended for patients with tumours greater than 4 cm in diameter, or tumours of any size in association with any of the following characteristics: multifocal disease, bilateral disease, extrathyroidal spread (pT3 and pT4a), familial disease, and those with clinically or radiologically involved nodes and/or distant metastases (R)
-
• Subtotal thyroidectomy should not be used in the management of thyroid cancer (G)
-
• Central compartment neck dissection is not recommended for patients without clinical or radiological evidence of lymph node involvement, provided they meet all of the following criteria: classical type PTC, below 45 years, unifocal tumour, less than 4 cm, no extrathyroidal extension on US (R)
-
• Patients with metastases in the lateral compartment should undergo therapeutic lateral and central compartment neck dissection (R)
-
• Patients with follicular tumours greater than 4 cm should be treated with total thyroidectomy (R)

Fig. 1 Flow diagram outlining management of papillary microcarcinomas. Multiple risk factors may tip the balance in favour of total thyroidectomy.
Post-operative management
After total or near total thyroidectomy patients should be commenced on suppressive doses of levothyroxine (2 µg/kg) or liothyronine 20 mcg tds in accordance with local protocols.
Calcium levels should be routinely checked within 24 hours and hypocalcaemia treated appropriately.
Thyroglobulin levels should be checked no earlier than six weeks after surgery.
All patients with thyroid cancer should be clinically staged using the TNM classification and also scored using one of the clinicopathological scoring systems to enable planned follow-up, identification of high risk patients and those who would benefit from radio-iodine therapy. In addition, all patients should have access to a thyroid cancer clinical nurse specialist and be given written information.
Persistent voice dysfunction should be investigated and referral to a specialised practitioner for assessment and speech therapy sought.
Patients with long-term hypocalcaemia (hypoparathyroidism) should have their calcium levels regularly monitored either in association with an endocrinologist or with their GP.
Following surgery, initial post-operative risk stratification for risk of recurrence can occur.
Low-risk patients have the following characteristics:
-
• No local or distant metastases
-
• All macroscopic tumours have been resected, i.e. R0 or R1 resection
-
• No tumour invasion of locoregional tissues or structures
-
• The tumour does not have aggressive histology (tall cell or columnar cell PTC, diffuse sclerosing PTC, poorly differentiated elements) or angioinvasion.
Intermediate-risk patients have any of the following characteristics:
-
• Microscopic invasion of tumour into the peri-thyroidal soft tissues (T3) at initial surgery
-
• Cervical lymph node metastases (N1a or N1b)
-
• Tumour with aggressive histology (tall cell or columnar cell PTC, diffuse sclerosing PTC, poorly differentiated elements) or angioinvasion.
High-risk patients have any of the following characteristics:
-
• Extrathyroidal invasion
-
• Incomplete macroscopic tumour resection (R2)
-
• Distant metastases. (M1)
Radioiodine (I131) ablation and external beam radiotherapy (EBR) in DTC
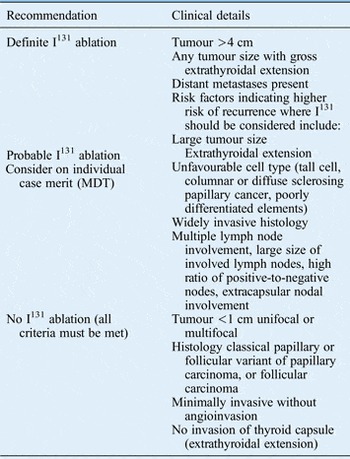
The current recommendations with regards to I131 ablation following total thyroidectomy are outlined in Table VII.
Table VII Indications for I131 ablation following total thyroidectomy for differentiated thyroid cancer

Patients should be prepared for I131 by having a low-iodine diet for one to two weeks prior to treatment. Recombinant TSH (rhTSH) therapy prior to I131 is preferable to thyroid hormone withdrawal, and is preferred by patients, providing they meet the following criteria: pT1 to T3, pN0 or NX or N1, and M0 and R0 (no microscopic residual disease). Pregnancy should be excluded prior to giving I131. A post-ablation scan should be performed after I131 when residual activity levels permit satisfactory imaging. Practically, this is generally 2–10 days following treatment.
Following I131, TSH should be suppressed to <0.1 mIU/l pending dynamic risk stratification at 9–12 months.
Adjuvant EBR should be considered in unresectable tumours in addition to I131 and where there is residual disease following surgical resection even if the residual tumour concentrates I131.
In the 9 to 12 months following surgery and I131 for DTC with an R0 resection, patients should undergo dynamic risk stratification (Table VIII). Patients are then categorised as having either an excellent response, an indeterminate response or an incomplete response.
Table VIII Dynamic risk stratification following treatment for DTC and TSH suppression targets for patients treated with total thyroidectomy and I131 ablation with R0 resection

* Assumes the absence of interference in the Tg assay. Tg = thyroglobulin; TSH = thyroid stimulating hormone; US = ultrasound
Monitoring Tg levels
Thyroglobulin monitoring is most effective following total or near total thyroidectomy and I131 and is an important modality in detecting residual or recurrent disease. Physicians should be aware that Tg estimations vary according to the assay method, the individual laboratory and the presence of anti-Tg antibodies and take these considerations into account when evaluating Tg levels in individual patients.
The patient should have their Tg levels checked at 6–12 monthly intervals. Rising Tg levels are highly suspicious of recurrent disease. Thyroglobulin evaluation is most effective following TSH stimulation, either by direct rhTSH stimulation or by withdrawal of thyroid hormone replacement.
Following total or near total thyroidectomy and I131 ablation, low-risk patients with undetectable Tg levels on TSH suppression should have a TSH-stimulated Tg assessment along with ultrasound of cervical nodes at 9–12 months following I131 ablation. If Tg levels remain undetectable following TSH stimulation, then future recurrent disease is highly unlikely and patients may revert to yearly Tg estimation whilst remaining on TSH suppression.
A rise in Tg may be suggestive of recurrent or residual disease, but is usually from a thyroid remnant. In low-risk patients, an expectant policy can be maintained and repeated TSH stimulated assessment performed, with the expectation that Tg levels will fall. Rising or persistently elevated Tg needs further evaluation.
The use of rhTSH-stimulated Tg estimation or rhTSH I131 therapy is necessary in the following cases: hypopituitarism, functional metastases (suppressing TSH), severe angina, advanced disease (frail patient) and history of psychiatric disturbance from hypothyroidism.
Recommendations
-
• I131 ablation or therapy should be carried out only in centres with appropriate facilities (R)
-
• Serum Tg should be checked in all post-operative patients with DTC, but not earlier than six weeks after surgery (R)
-
• Patients who have undergone total or near total thyroidectomy should be started on levothyroxine 2 µg/kg or liothyronine 20 mcg tds after surgery (R)
-
• The majority of patients with a tumour more than 1 cm in diameter, who have undergone total or near-total thyroidectomy, should have I131 ablation or therapy (R)
-
• A post-ablation scan should be performed 3–10 days after I131 ablation (R)
-
• Post-therapy dynamic risk stratification at 9–12 months is used to guide further management (G)
Persistent and recurrent disease, locoregional recurrence and distant metastases
Potentially resectable disease is best managed by surgery (including local cervical nodes and soft tissue disease in the neck), followed by I131. Residual disease not amenable to resection or resistant to I131 therapy is best treated with high dose palliative EBR.
Therapeutic central compartment, with or without lateral compartment, nodal clearance should therefore be performed for all persistent or recurrent disease confined to the neck. Impalpable nodes greater than 5–8 mm seen on USS or cross-sectional imaging following I131 therapy should be considered for removal. Removing nodes less than 5–8 mm has not be shown to be of benefit.
Where technically feasible, tumours invading the aero-digestive tract should be resected in combination with radiotherapy. Outcome is very dependent on completeness of resection and preservation of function. Great care should therefore be taken in the selection and discussion of such patients at the MDT.
Distant metastases develop in 5–23 per cent of patients with DTC. Sites not amenable to surgical resection should be treated with I131 therapy. Long-term survival may be expected in patients whose tumours take up I131. Distant metastases are usually seen in the lungs and bones. There is no maximum limit to the cumulative dose of I131 that patients with persistent disease may receive and pulmonary fibrosis appears to be a rare side effect. Surgical resection of bony metastases should be considered (especially in patients below 45 years of age). Metastases not cured by I131 should be treated with EBR. Other modalities such as intra-arterial embolisation, pamidronate infusion, radiofrequency ablation or vertebroplasty may be considered in cases of painful lesions.
Recommendations
-
• Potentially resectable recurrent or persistent disease should be managed with surgery whenever possible (R)
-
• Distant metastases and sites not amenable to surgery, which are iodine avid should be treated with I131 therapy (R)
Long-term follow-up
Lifelong follow-up of DTC is recommended to monitor for late recurrence (often treatable and curable), effects of long-term TSH suppression (atrial fibrillation and osteoporosis) and late side effects of I131. Clinical examination and history, Tg determination, TSH suppression and where necessary calcium monitoring should all be performed. Ultrasound scanning as per established protocols may also be undertaken.
Recommendations
-
• Long-term follow-up for patients with DTC is recommended (G)
-
• Follow-up should be based on clinical examination, serum Tg and TSH assessments (R)
Medullary thyroid cancer
Introduction
Medullary thyroid cancer (MTC) is a rare cancer (approximately 1–3 per cent of all thyroid cancer cases). All cases should be referred for surgical treatment to the designated cancer centre of the Thyroid Cancer Network. Twenty-five per cent of MTC cases are familial (MEN2A, MEN2B and familial non-MEN MTC). Genetic screening (RET mutation testing) of all patients is mandatory and the assessment, investigation and treatment of family members at potential risk requires a multidisciplinary approach within the cancer centre.Reference Niederle, Sebag and Brauckhoff 7
Clinical presentation
Patients usually present clinically with a thyroid nodule or neck mass with or without cervical lymphadenopathy (in the same fashion as with DTC). History however, may reveal other symptoms such as flushing, loose stools or diarrhoea (which suggest MTC) and is vitally important in determining a potential familial element. FNAC may be diagnostic (when combined with calcitonin staining in suspicious cases), but often is reported as Thy 3.
Investigation
When MTC is suspected (or proven) patients must undergo the following investigations prior to surgeryReference Wells, Asa, Dralle, Elisei, Evans and Gagel 8 :
-
• Calcitonin and CEA levels
-
• Twenty-four-hours urine estimation of catecholamines and nor metanephrines (or plasma nor metanephrines) to identify or exclude phaeochromocytoma
-
• Serum calcium and parathyroid hormone (PTH) to identify or exclude hyperparathyroidism
-
• CT, MRI or USS of the neck are indicated as they may help guide the extent of surgical resection at initial surgery
-
• RET proto-oncogene mutational analysis should be performed after surgery once diagnosis is established, even in the absence of a familial history.
Staging
TNM staging for MTC follows the same criteria as for DTC (Table IX).
Recommendations
-
• Patients with suspected MTC should be investigated with calcitonin and CEA levels, 24 hours catecholamine and nor metanephrine urine estimation (or plasma free nor metanephrine estimation), serum calcium and PTH (R)
-
• Relevant imaging studies are advisable to guide the extent of surgery (R)
-
• RET proto-oncogene analysis should be performed after surgery (R)
Table IX Group staging for medullary thyroid cancer

Management-surgery for MTC
All patients with MTC should undergoReference Wells, Asa, Dralle, Elisei, Evans and Gagel 8 :
-
• Total thyroidectomy and central compartment node clearance (level VI). This should be performed even in the presence of disseminated metastases to control local disease.
-
• In the presence of central compartment lymph node metastases, ipsilateral prophylactic neck dissection is recommended as up to 70 per cent of patients will have lateral nodal metastases.
-
• Patients with clinically involved lateral compartment nodes should have a therapeutic lateral neck dissection to eradicate local disease.
-
• All T2–T4 tumours should also undergo prophylactic bilateral selective neck dissection IIa–Vb.
-
• Intra-thoracic disease below the level of the brachiocephalic vein should be resected via sternotomy where feasible.
-
• Prophylactic thyroidectomy should be offered to RET-positive family members. Timing and extent of surgery are dependent on genotype (codon mutation), the calcitonin level and age at detection of RET positivity.
Persistent or recurrent MTC
Calcitonin levels are most informative six months after initial surgery. It is important to distinguish persistent locoregional disease (following either inadequate initial surgery or local lymph node metastases) from distant disease.
Early local recurrence following adequate local surgery (total thyroidectomy and level VI nodes) is unusual. The likely source of raised calcitonin in this circumstance is the lateral compartment cervical nodes, i.e. persistent disease. When indicated, re-operation including further central compartment surgery and lateral neck node dissection should be performed. The primary aim should always be to control local disease.
CT, MRI, USS, selective arteriography, I131-metaiodobenzylguanidine, 18Fluoro-deoxy-glucose positron emission tomography, In111-octreotide and direct laparoscopic visualisation of the liver may all be useful in identifying the source of a raised calcitonin, but their use in patients with calcitonin levels <400–500 pg/ml is unlikely to identify metastases. When indicated, isolated metastases should be considered for surgical resection.
Recommendations
-
• All patients with known or suspected MTC should have serum calcitonin and biochemical screening for phaeochromocytoma pre-operatively (R)
-
• All patients with proven MTC >5 mm should undergo total thyroidectomy and central compartment neck dissection (R)
-
• Patients with lateral nodal involvement should undergo selective neck dissection (IIa–Vb) (R)
-
• Patients with central node metastases should undergo ipsilateral prophylactic lateral node dissection (R)
-
• Prophylactic thyroidectomy should be offered to RET-positive family members (R)
-
• All patients with proven MTC should have genetic screening (R)
Radiotherapy and chemotherapy
Radiotherapy is of use in controlling local symptoms in patients with inoperable disease and improving the relapse-free rate following central or lateral compartment surgery where residual disease is present macroscopically or microscopically.
Tyrosine kinase inhibitors can be effective in controlling symptoms in patients with metastatic disease.
Somatostatin analogues may be effective in alleviating the unpleasant gastrointestinal symptoms that patients with advanced cases of MTC experience.
Recommendations
-
• Radiotherapy may be useful in controlling local symptoms in patients with inoperable disease (R)
-
• Chemotherapy with tyrosine kinase inhibitors may help in controlling local symptoms (R)
Follow-up
Lifelong follow-up is recommended for all patients with MTC. Screening should include calcitonin and CEA. Thyroid-stimulating hormone suppression is not necessary. Rising calcitonin levels should trigger investigations to identify potentially treatable metastatic disease.
Anaplastic thyroid cancer
The prognosis of patients with anaplastic thyroid cancer (ATC) is poor. Many patients present with a history of a rapidly enlarging thyroid mass in a long-standing goitre. Diagnosis can be established by fine needle aspiration or core biopsy. Core biopsy will help differentiate ATC from thyroid lymphoma which can present in a similar manner.
Total thyroidectomy may be curative for very small cancers. In more advanced disease surgery may be of benefit if R0/R1 resection is achievable.Reference Smallridge, Ain, Asa, Bible, Brierley and Burman 9 External beam radiotherapy and chemotherapy may be used as adjuvant treatments in patients with R0/R1 resection and no evidence of distant disease. ‘Debulking’ surgery should be avoided when complete resection cannot be achieved. Palliative chemoradiation may be of some value in selected cases. Palliative care has a principal role in management of these patients.
Recommendations
-
• Initial assessment should focus on identifying the small proportion of patients with localised disease and good performance status, who may benefit from surgical resection and other adjuvant therapies (G)
-
• The surgical intent should be gross tumour resection and not merely an attempt at debulking (G)