Introduction
Tracheoesophageal fistula is a pathological condition characterised by an abnormal connection between the trachea and the oesophagus, which may be congenital or acquired in nature. Acquired tracheoesophageal fistula, seen more commonly in adults, occurs due to benign or malignant conditions.Reference Kim, Khemasuwan, Diaz-Mendoza and Mehta1 Carcinoma of the oesophagus, larynx, trachea, lung or thyroid, as well as chemo-radiation therapy, steroid use and stent placement, contribute to approximately half of all acquired cases.Reference Kim, Khemasuwan, Diaz-Mendoza and Mehta1 An iatrogenic tracheoesophageal shunt may also be the preferred method for voice rehabilitation following total laryngectomy, with little need for training and no equipment requirement.Reference Morimatsu, Yonezawa, Matsui, Iwae and Sakakibara2 Rarely, Crohn's disease involving the oesophagus may lead to a tracheoesophageal fistula.Reference Asarian, Esan and Adeoye3
Treatment of a tracheoesophageal fistula involves a multidisciplinary approach, including pulmonology, gastroenterology, intervention radiology, and head and neck reconstructive specialists. A tracheoesophageal fistula may paradoxically be a sequelae of tumour progression or treatment. Although stent placement has been the mainstay of treatment, it is associated with pressure necrosis and propagation of fistulae, and is usually reserved for patients with a mean survival expectancy of one to six weeks.Reference Herth, Peter and Baty4 Conservative techniques like fibrin glue and micromatrices have limited efficacy, especially in large fistulae.Reference Kim, Khemasuwan, Diaz-Mendoza and Mehta1 Such conservative attempts to close the two layers are ineffective, and healthy vascularised tissue needs to be interposed to prevent recurrence. The various flap options have traditionally included pectoralis major, latissimus dorsi, sternohyoid or platysma pedicled muscle/myocutaneous flaps; however, these are bulky and may cause further stomal obstruction. Other options, like a thymus pedicle flap, pleural flap, diaphragmatic flap and azygous flap, have been described for lower fistulae, but require sternotomy.Reference Fukumoto, Matsunaga, Shishido, Amisaki, Kono and Murakami5
Knowledge of angiosomes and perforator anatomy, combined with advances in microvascular surgery, have enabled the recruitment of thin, pliable tissue from the surrounding trunk, with minimal donor site morbidity.Reference Koshima and Soeda6 Vesely et al., in 2007, described in detail the anatomy and applications of the internal mammary artery perforator flap after cadaver studies.Reference Vesely, Murray, Novak, Gullane and Neligan7 However, there is still limited literature on its applications, as it is not reported extensively. The use of an islanded internal mammary perforator flap in patients with tracheoesophageal fistula is discussed here.
Materials and methods
Out-patient and operative records of all patients who underwent tracheoesophageal fistula closure with an internal mammary artery perforator flap were reviewed. The following data were collected: patient demographics, time interval since completion of radiotherapy, characteristics of the perforator chosen, size of skin paddle, technique of flap inset, donor site closure method and any post-operative complications.
Surgical technique
Intra-operative, hand-held Doppler assessment was performed, 1–2 cm lateral to the sternal border. All anterior intercostal perforators audible in the first four spaces were marked. The left side was chosen for simultaneous flap elevation by the plastic surgeon, while the head and neck surgeon operated from the right side, for surgical ease. By planning in reverse, the flap was marked with the eccentrically located pedicle (Figure 1).
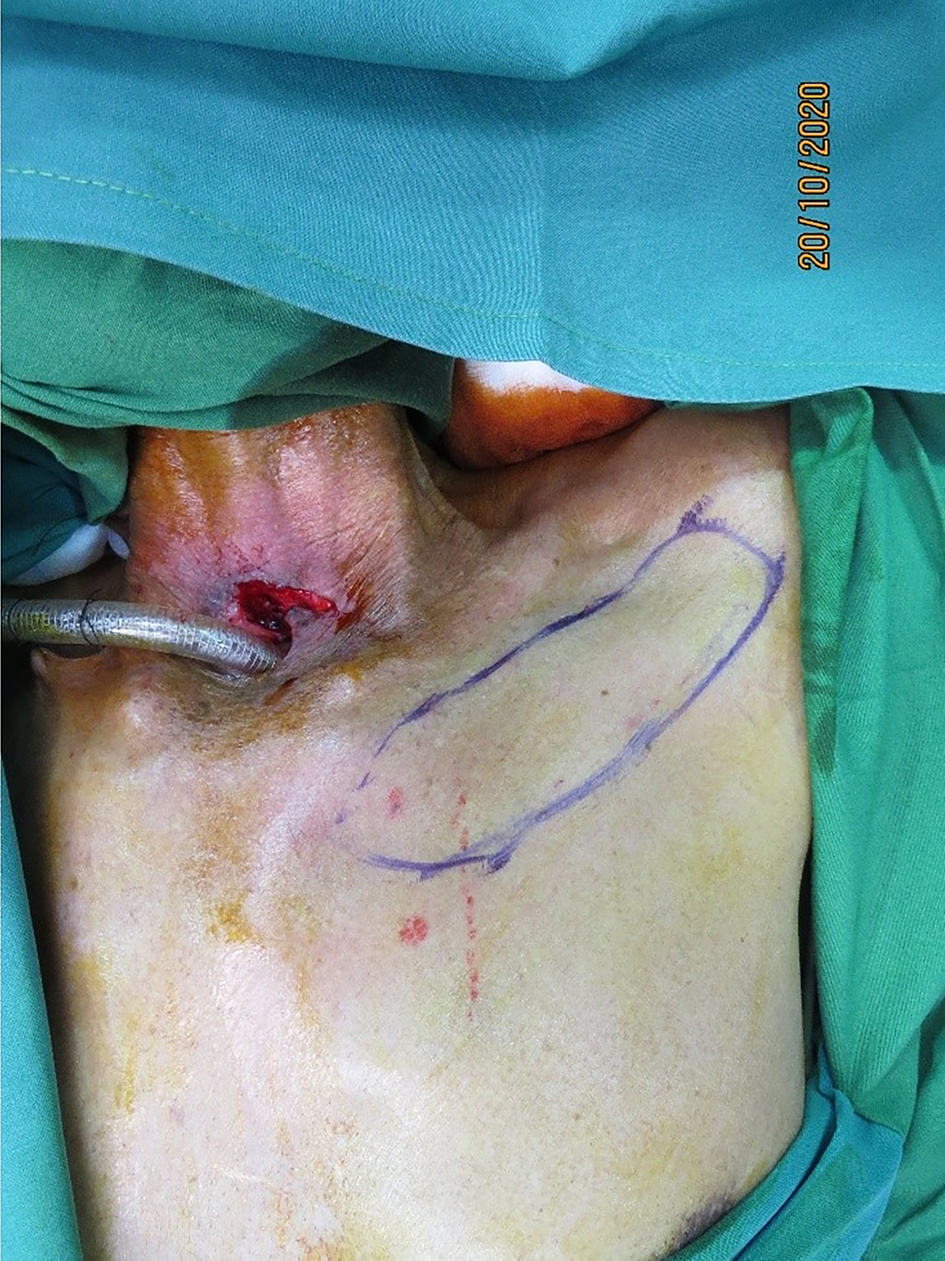
Figure 1. Left second internal mammary artery perforator flap marked below the clavicle.
The flap was elevated from lateral to medial, suprafascially, up to 2–3 cm near the sternal edge, till the pedicle was visualised (Figure 2), and thereafter subfascially. The adequacy of the selected perforator was confirmed by visible pulsations. Intramuscular dissection was carried out by splitting the fibres of the pectoralis major and intercostals, and any sternal or muscular branches were clipped. A pedicle length of 2–2.5 cm was usually achieved. The flap was islanded on the chosen pedicle, tunnelled, and the intervening segment de-epithelised (Figure 3). The accompanying intercostal nerve was sacrificed, leading to an insensate flap. If the intervening neck skin was of poor quality due to prior radiation, the skin bridge was incised (Figure 4). Simultaneous separation and dissection of the pathological connection was performed by the head and neck oncologist.
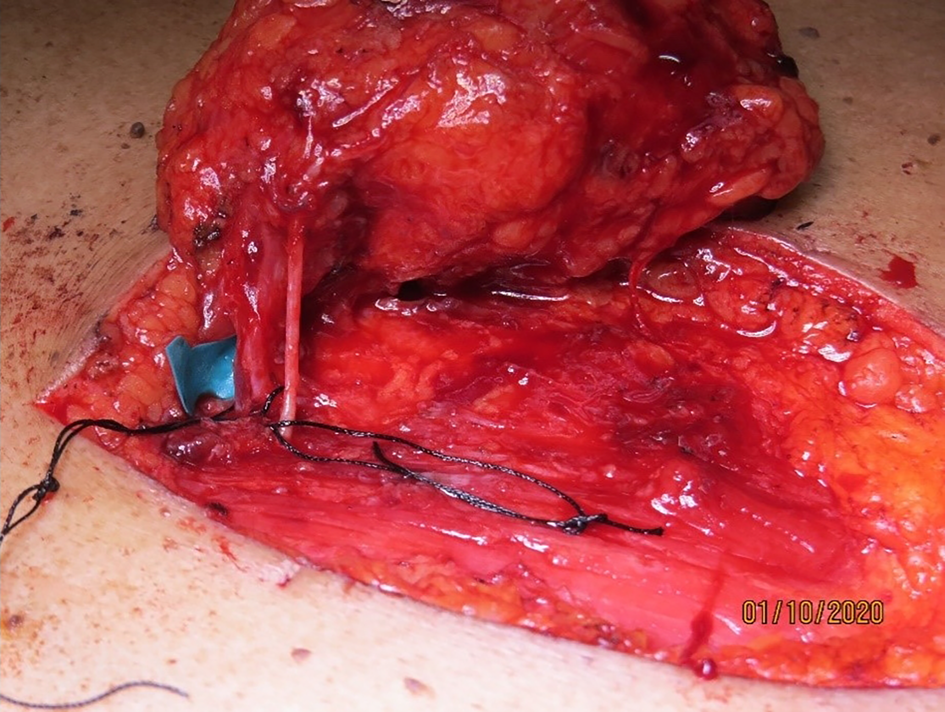
Figure 2. Dissected internal mammary artery perforator pedicle through the pectoralis major fibres, with the accompanying nerve, with a pedicle length of 2.5 cm gained.
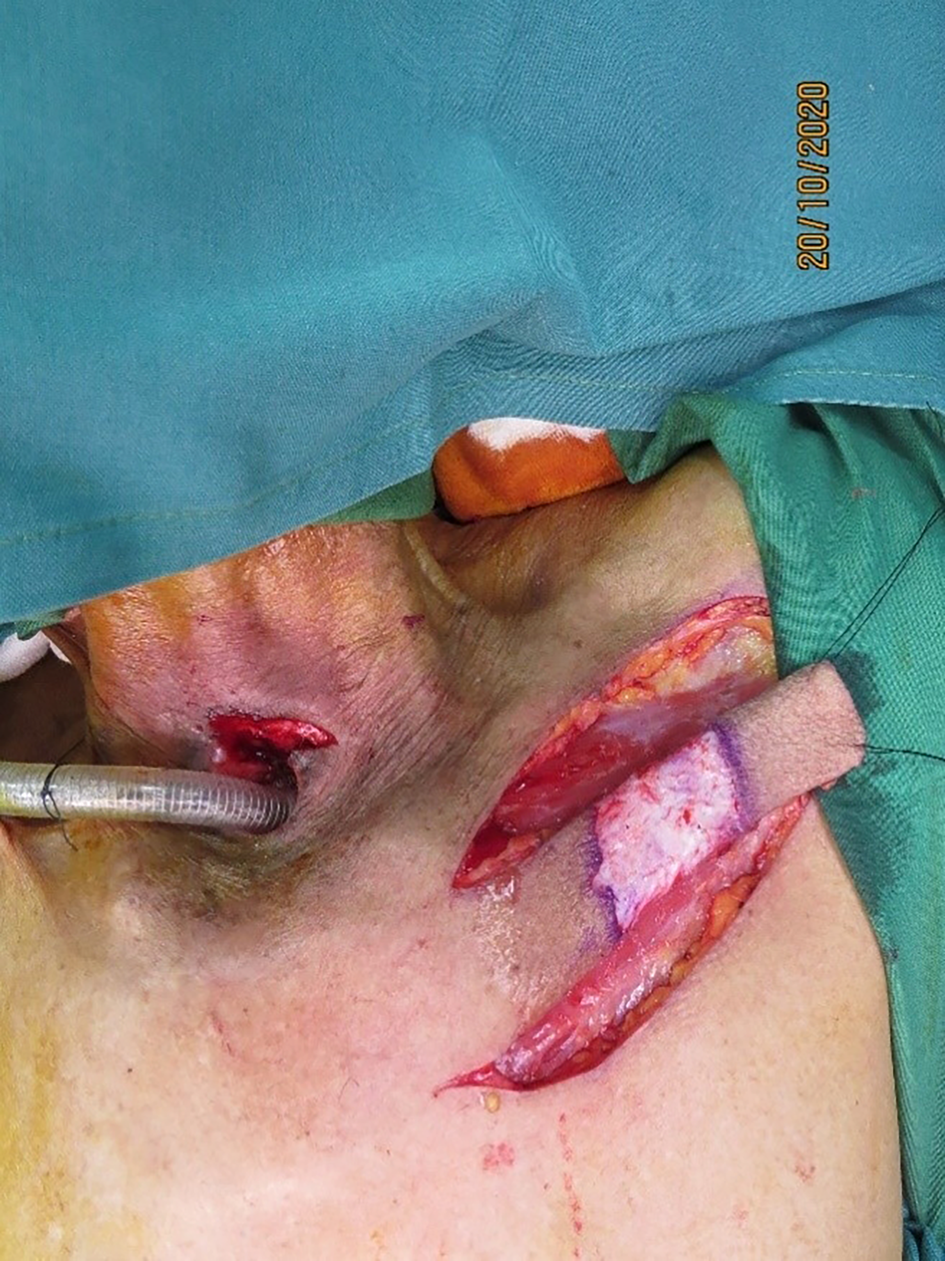
Figure 3. Flap elevation with de-epithelised intervening segment.
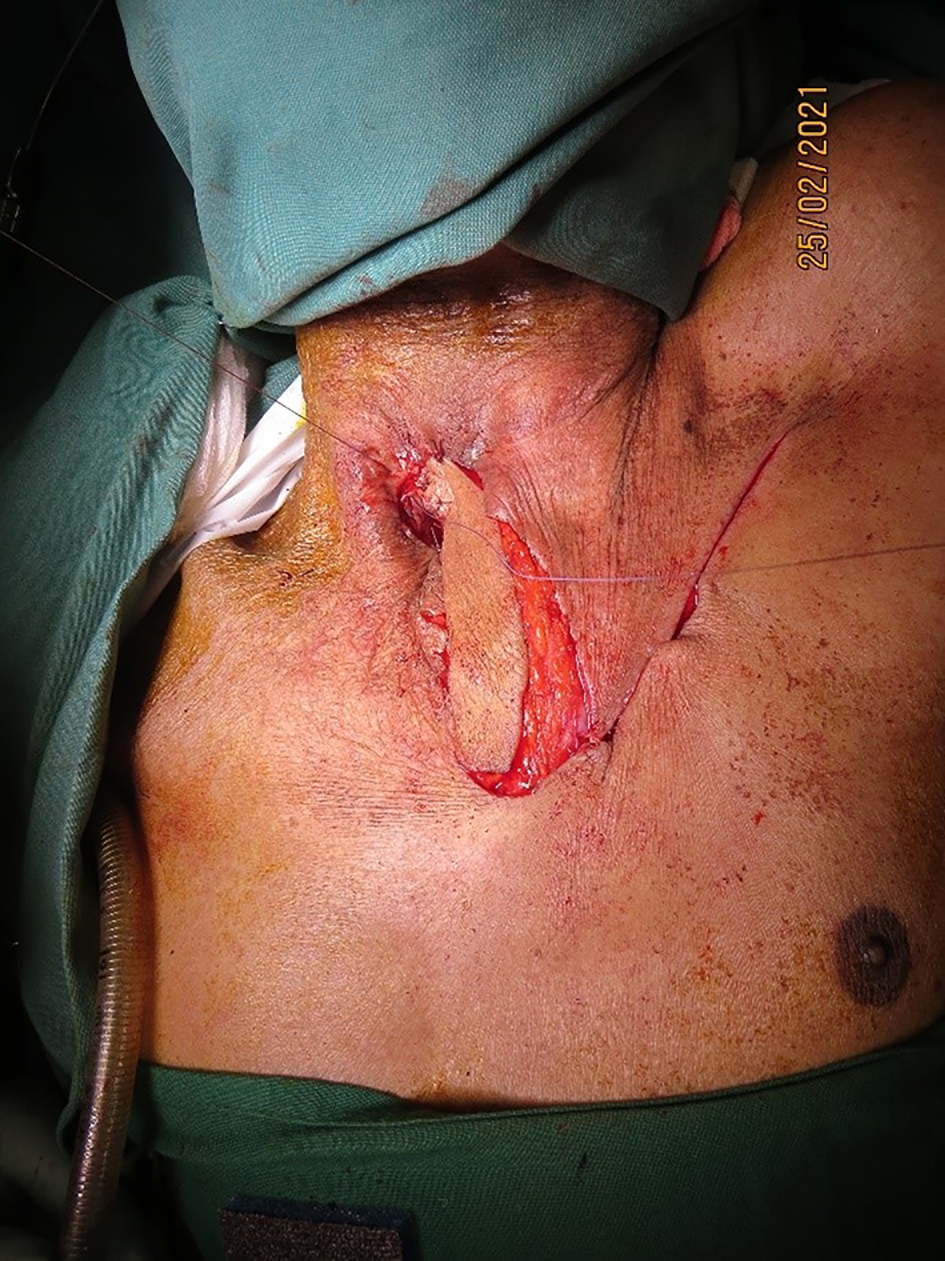
Figure 4. Intervening neck skin incised in view of prior radiation causing severe dermal atrophy.
The flap was inset with the fascial surface on the oesophageal suture line and skin surface along the tracheal suture line. The donor site was closed by wide undermining, primarily, in two layers, after securing haemostasis (Figure 5).
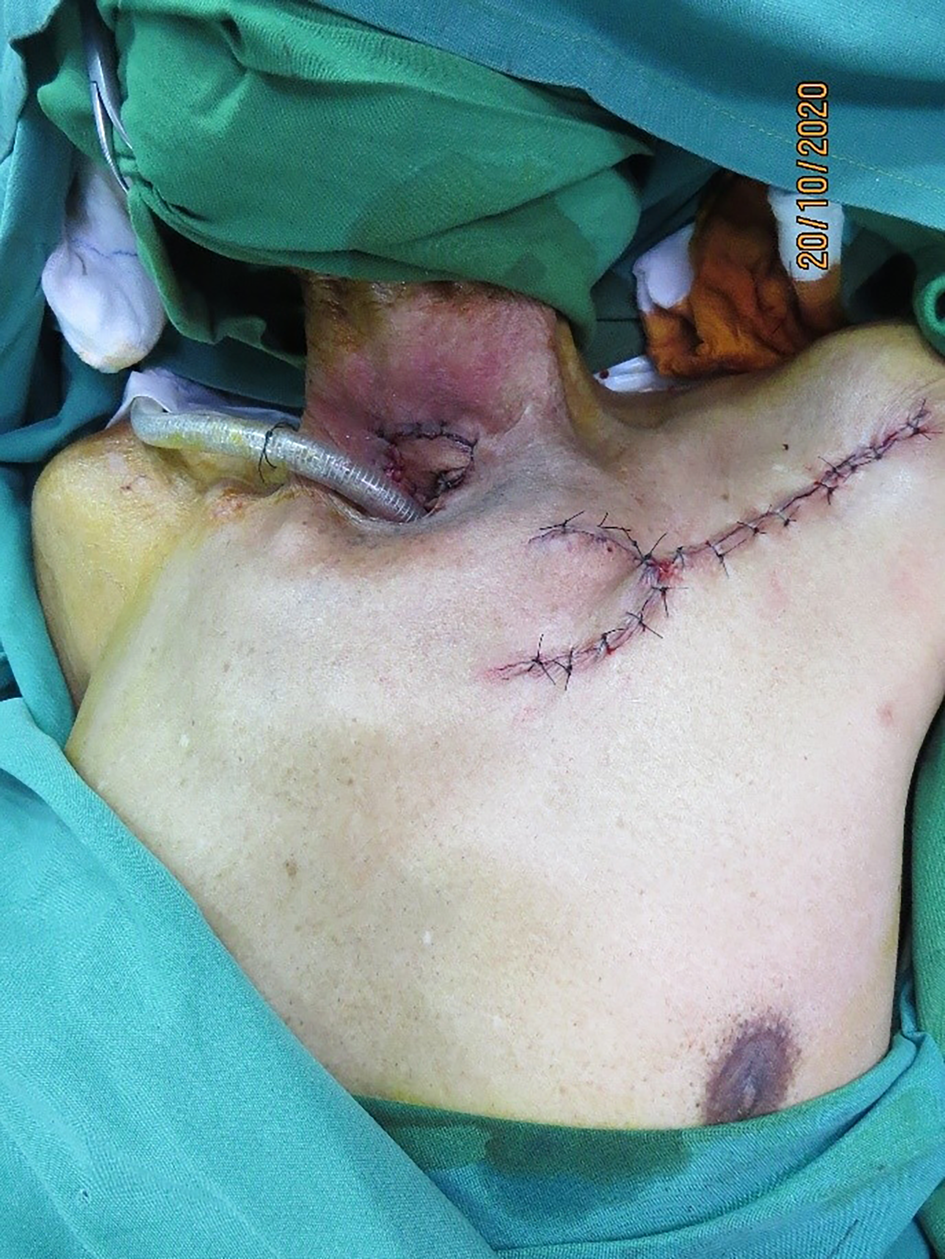
Figure 5. Complete flap inset with primarily closed donor site.
Results
Four patients, all males, underwent tracheoesophageal fistula closure between September 2017 and February 2021. The average age of the patients was 60.75 years (range, 47–75 years). The time interval from completion of radiotherapy varied from one to five years. In all patients, the flap was based on the left-sided second internal mammary artery perforator, and the donor site was closed primarily, without any standing cone deformity. The flap was subcutaneously tunnelled in three patients, with the intervening skin bridge being de-epithelised (Table 1). One patient underwent incision of the scarred chest skin for flap inset. Costal cartilage resection was not performed in any patients.
Table 1. Patient characteristics and surgical details
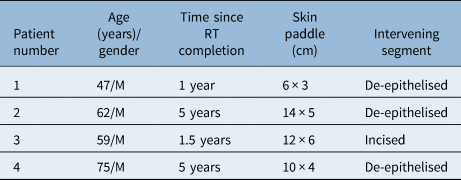
RT = radiotherapy; M = male
All patients were followed up for a minimum of six months, during which there were no complications of persistent tracheoesophageal leak, venous congestion, flap loss or donor site scar-related issues (Figure 6).
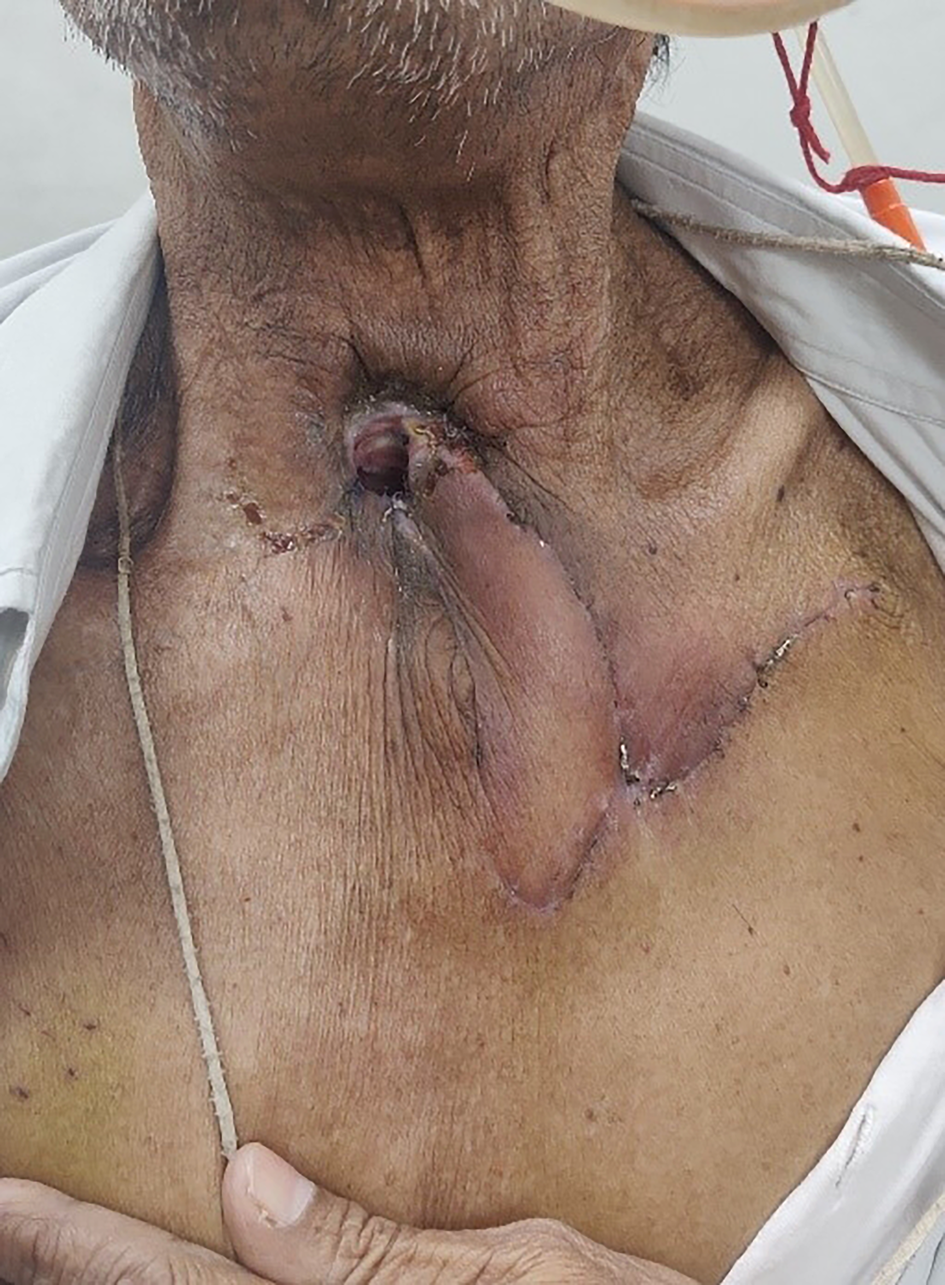
Figure 6. Post-operative follow-up view after two weeks, showing a healthy flap with no donor complications.
Discussion
Separation of any fistulous tract requires tract isolation, division, interposition of vascular tissue and tension-free layered closure of the lining elements. These steps are critical to prevent a fibrotic scar around the site, and thus avoid recurrence.Reference Murono, Ishikawa, Nakanishi, Endo, Kondo and Wakisaka8
Closure of a tracheoesophageal fistula is challenging, particularly in an irradiated field.Reference Marsden, Shukla and Grinsell9 A thin and pliable flap is necessary given the tubular configuration of the pharynx and trachea, and the narrow access of this anatomical region, which is limited by the sternum and clavicles. Historically, muscle and myocutaneous flaps – the pectoralis major and latissimus dorsi – were used for resurfacing lower face and neck defects. However, these were bulky, with the potential to cause tracheostomal obstruction and added donor site functional loss.Reference Schellekens, Hage, Paes and Kon10
As fasciocutaneous composite tissue, the internal mammary artery perforator flap fulfils these criteria and has become our first choice in this situation. It may be used to close the most commonly found tracheoesophageal fistula, as its superior limit is the lower third of the face.
The mammary artery pectoral flap, originally described by von Joseph in 1931,Reference Joseph11 was based on the third and fourth anterior intercostal perforators. This was followed by Conley in 1960, who described the anterior chest flap, with the medial pedicle running infero-obliquely from the sternum, based on the internal mammary perforators.Reference Conley12 Bakamjian, in 1965, described ‘rotating an oblong rectangular flap of skin from the pectoral region into the neck and inverting it to form a hollow epidermal tube based on the perforating branches of internal mammary vessels’ for pharyngoesophageal reconstruction.Reference Bakamjian13 He called it the deltopectoral flap; however, he did not visualise the perforators. Additionally, the donor site required skin graft for closure. With the advent of microsurgical technology and its widespread use, this chest wall flap fell out of favour, only to be used as a salvage option.
The concept of perforasomes, introduced in 1989 by Koshima and Soeda, ushered an era of defining composite tissues based on named and unnamed perforators.Reference Koshima and Soeda6 This led to the description of an internal mammary artery perforator island flap for anterior neck reconstruction by Yu et al. in 2006,Reference Yu, Roblin and Chevray14 and Vesely et al. in 2007.Reference Vesely, Murray, Novak, Gullane and Neligan7 We use it as a first choice in tracheoesophageal fistula closure, in post-radiated laryngeal carcinoma patients.
The angiosomal territory of the internal mammary artery has been well documented in studies on living patients and cadavers.Reference Schellekens, Paes, Hage, van der Wal, Bleys and Kon15 It encompasses an area from the clavicle to the level of the ninth rib, and from the midline to the anterior axillary line, with a variable area in the superolateral region being occupied by the thoracoacromial angiosome. The cutaneous perforators are located 13–14 mm from the lateral sternal border, with diameters ranging from 1 to 1.5 mm. They pass through the medial fibres of the pectoralis major. The perforating vessels are usually found in the first five intercostal spaces, but are more frequently found in the second (68.9 per cent) and third (27.2 per cent) intercostal spaces. The second perforator was most often the largest in both sexes, being generally greater than 0.8 mm. In females, the third and fourth perforators also tend to be large, as they contribute to the arterial supply of the breast.Reference Vesely, Murray, Novak, Gullane and Neligan7,Reference Schellekens, Paes, Hage, van der Wal, Bleys and Kon15 However, the internal mammary perforators have been shown to vary in size and dominance between patients and chest side. Palmer and Taylor found that there was a single dominant perforator in 85 per cent of cases, which was at least twice the size of any other perforator, while in the other 15 per cent there was co-dominance, with two perforators being of equal size. The diameter of the dominant perforator is 0.5–1.2 mm, and that of the accompanying vein is 1.5–3.2 mm.Reference Palmer and Taylor16
Mirghani et al., in their retrospective 2013 study of 12 internal mammary artery perforator flaps, described removing the second or third rib cartilage immediately superior to the selected internal mammary artery perforator for enhanced exposure.Reference Mirghani, Leymarie, Amen, Qassemyar, Leclère and Kolb17 They then ligated the internal mammary artery and vein distal to the origin of the selected internal mammary artery perforator, to increase the total length of the flap pedicle and improve its arc of rotation.Reference Mirghani, Leymarie, Amen, Qassemyar, Leclère and Kolb17 In our series, sacrificing the distal inferior mesenteric artery vessels and costochondral resection has not been necessary. Dissecting the pedicle through its intramuscular course alone, enabled adequate reach.
The internal mammary artery perforator flap has several advantages over the deltopectoral flap: the ability to be islanded, the avoidance of ‘dog-ear formation’ and primary closure of the donor site. Pedicle twisting or kinking has not been reported.
Resection of costal cartilage to gain pedicle length has been reported to cause pneumothorax rarely.Reference Schellekens, Hage, Paes and Kon10 Although issues with flap venous congestion have been described, we did not encounter it. There were no complications of flap retraction, suture line dehiscence or donor site scar hypertrophy.
• Management of pathological tracheoesophageal fistulae is complicated
• Non-surgical measures for temporary control are usually reserved for palliation only
• Current knowledge of angiosomal concepts combined with microvascular expertise have added new perspectives to tackle this difficult condition
• An islanded internal mammary artery perforator flap provides reliable, pliable tissue for interposition, with no donor site complications
This technique may similarly be employed for the management of tracheoesophageal fistulae of other aetiology – like trauma or Crohn's disease – as long as the pedicle and flap are outside the zone of injury. However, our experience is limited to radiated patients with laryngeal carcinoma.
Conclusion
The islanded internal mammary artery perforator flap provides high-quality, thin, pliable skin, which, in combination with microsurgical dissection expertise, offers a reliable option for the closure of difficult-to-manage tracheoesophageal fistulae.
Competing interests
None declared









