Introduction
Certain slug species are considered pests of agriculture, horticulture, and floriculture worldwide (Barker Reference Barker2002; Koslowski Reference Kozlowski2012). Agricultural and horticultural sites, commercial nurseries, greenhouses, and residential gardens provide ideal habitats for slugs to thrive as they often provide moist and shaded environments (Douglas & Tooker Reference Douglas and Tooker2012; Koslowski Reference Kozlowski2012). When slug density is high, large-scale no-tillage crops such as wheat, barley, oats, rye, corn, canola, tobacco, soybean, alfalfa, and leguminous forages often suffer extensive damage (Douglas & Tooker Reference Douglas and Tooker2012; South Reference South1992). The damage is not limited to large-scale arable crops but may also include many other crops including strawberries, cabbage, leeks, potatoes, and carrots (Thomas Reference Thomas2010). In severe cases, complete germination failure is possible due to grain hollowing even before the seeds germinate. Further, herbivory on the seedlings of arable plants can lead to a reduced crop stand and reduced yield. Pestiferous slug species are often inadvertently introduced to new areas through poor quarantine practices during the transport of agricultural and horticultural commodities and human travel (Cowie et al. Reference Cowie, Dillon, Robinson and Smith2009; Darrigran et al. Reference Darrigran, Agudo-Padrón, Baez, Belz, Cardoso, Carranza, Collado, Correoso, Cuezzo, Fabres and Gutiérrez Gregoric2020; Howlett Reference Howlett2012; Schurkman et al. Reference Schurkman, Tandingan De Ley, Anesko, Paine, Mc Donnell and Dillman2022b). Rapid adaptability and spread in new settings in the absence of pressure from natural predators and competitors have led to some pest slug species establishing in many new parts of the world, from the tropics to temperate regions (Howlett Reference Howlett2012). Pestiferous slug genera in temperate climates include Deroceras, Milax, Arion, and Limax. For example, Arion vulgaris, Moquin-Tandon, 1885 and Deroceras reticulatum are among the most pestiferous slug species in Europe (Howlett Reference Howlett2012). Certain European slug species have also been introduced to temperate regions such as New Zealand, Australia, and South Africa (Howlett Reference Howlett2012).
The dominant method for controlling slugs worldwide is the application of agrochemicals, e.g., methiocarb, metaldehyde, and iron phosphate (South Reference South1992). However, growing public opposition to pesticides and appreciation of eco-friendly agricultural practices favor the use of biopesticides and biocontrol agents in controlling slug populations. These can provide higher specificity and sometimes are more efficient pest management tools than other practices (Jaffuel et al. Reference Jaffuel, Půža, Hug, Meuli, Nermuť, Turlings, Desurmont and Campos-Herrera2019; Mc Donnell et al. Reference Mc Donnell, Colton, Howe and Denver2020). Nematodes, some of which are parasitic, offer a potential solution due to their natural associations with terrestrial gastropods (Wilson & Grewal Reference Wilson, Grewal, Grewal, Ehlers and Shapiro-Ilan2005). Previous surveys conducted in Germany, France, Slovenia, Bulgaria, the USA, Australia, Africa, and the UK found nematodes associated with slugs belonging to seven families, namely Agfidae, Alloionematidae, Angiostomatidae, Cosmocercidae, Diplogasteridae, Mermithidae, and Rhabditidae (Ross et al. Reference Ross, Ivanova, Hatteland, Brurberg and Haukeland2016). The most well-known is a facultative parasite of slugs, Phasmarhabditis (Family Rhabditidae), a genus of bacterial-feeding soil-dwelling nematodes (Wilson & Grewal Reference Wilson, Grewal, Grewal, Ehlers and Shapiro-Ilan2005). Phasmarhabditis currently contains eighteen nominal species (Ivanova et al. Reference Ivanova, Clausi, Leone and Spiridonov2022; Rae et al. Reference Rae2023), four of which have been shown to be pathogenic to slugs (Holley Reference Holley2020; Ivanova & Spiridonov Reference Ivanova and Spiridonov2021; Schurkman et al. Reference Schurkman, Tandingan De Ley and Dillman2022a; Wilson & Grewal Reference Wilson, Grewal, Grewal, Ehlers and Shapiro-Ilan2005): P. hermaphrodita (Schneider, 1859); P. papillosa, (Schneider Reference Schneider1866), Andrássy, Reference Andrássy1983; P. neopapillosa, (Mengert in Osche, Reference Osche1954), Andrássy Reference Andrássy1983; and P. californica, (Tandingan De Ley et al., Reference Tandingan De Ley, Holovachov, Mc Donnell, Bert, Paine and De Ley2016). Among them, P. hermaphrodita has been widely used as a biocontrol agent since it was commercialized in 1994 (Rae et al. Reference Rae, Verdun, Grewal, Robertson and Wilson2007). The product was launched under the trade name Nemaslug® and is currently only commercially available in 15 European countries, owing to the natural occurrence of P. hermaphrodita in those regions (Laznik et al. Reference Laznik, Majić, Trdan, Malan, Pieterse and Ross2020; Mc Donnell et al. Reference Mc Donnell, Colton, Howe and Denver2020; Rae et al. Reference Rae, Verdun, Grewal, Robertson and Wilson2007). This nematode is lethal to many slug species (in the families Arionidae, Milacidae, Limacidae, and Vaginulidae), particularly to D. reticulatum, arguably the most damaging slug species throughout the world (Howlett Reference Howlett2012; Rae et al. Reference Rae, Verdun, Grewal, Robertson and Wilson2007). Since its commercialization, this nematode has been discovered in geographic areas other than Europe, such as Iran, Egypt, New Zealand, the USA, Norway, and China (De Ley et al. Reference De Ley, Holovachov, Mc Donnell, Bert, Paine and De Ley2016; Howlett Reference Howlett2012; Rae et al. Reference Rae, Verdun, Grewal, Robertson and Wilson2007). Similarly, P. californica was recently discovered in California and Oregon (De Ley et al. Reference De Ley, Holovachov, Mc Donnell, Bert, Paine and De Ley2016; Howe et al. Reference Howe, Ha, Colton, De Ley, Rae, Ross, Wilson, Nermut, Zhao, Mc Donnell and Denver2020) and has recently been commercialized as Nemaslug 2.0 in the U.K. market (Mc Donnell et al. Reference Mc Donnell, Howe and Denver2023).
Canada had no previous record of Phasmarhabditis in the region until a recent discovery of a Canadian strain of P. californica, which was reported from a single slug (Arion rufus) collected from the exterior grounds of a local nursery in Edmonton (Brophy et al. Reference Brophy, Howe, Denver and Luong2020a; Brophy et al. Reference Brophy, Mc Donnell, Howe, Denver, Ross and Luong2020b). However, isolating a Canadian strain of P. californica from a single slug specimen is not sufficient evidence to confirm that the nematode is established locally. Thus, the goal of our study was to complete a more comprehensive survey including agricultural sites, commercial nurseries, and greenhouses in Alberta, Canada for pest slug species and their associated nematodes. These results provide valuable information to agro-practitioners in the region about pest slug species that are of agricultural and horticultural importance (residential gardens were not considered economically important sites in this survey). Our second objective was to confirm if the novel Canadian P. californica strain naturally occurs in agricultural and horticultural sites in Alberta, Canada.
Materials and methods
Slug collection
Slugs were collected from field margins and the adjacent vegetation stripes of three major agricultural sites in Alberta (Figure 1) from August to September 2021, a period corresponding to peak slug activity (Brophy et al. Reference Brophy, Mc Donnell, Howe, Denver, Ross and Luong2020b). In addition, ten commercial greenhouses and nurseries in Alberta were surveyed from June to September 2021 (Figure 1). Surveys were conducted in potential slug microhabitats such as soil surface, leaf litter, crop foliage, near the roots, under nursery pots, rocks, and logs, and in the marginal vegetation; on certain occasions when the surface soil was dry, the slugs were collected 1–5 cm deep in the soil. Each site was searched for a maximum of 30 minutes/person (2–4 people), and the slugs were handpicked and transferred into plastic containers with a perforated lid and lined with damp paper towels to prevent desiccation. Every site was visited at least twice during the survey period. Slugs collected from different sampling sites were put into separate containers to avoid any cross-contamination. Each slug species was photographed and identified using available taxonomic keys (Grimm et al. Reference Grimm, Forsyth, Schueler and Karstad2009; Mc Donnell et al. Reference McDonnell2009; Perez et al. Reference Perez, Cordeiro and Coppolino2008; https://idtools.org/mollusk). Molecular diagnosis for slugs was performed when we could not reliably make an identification using morphological traits, or if the samples contained immature slugs that were difficult to identify solely on external morphologies.
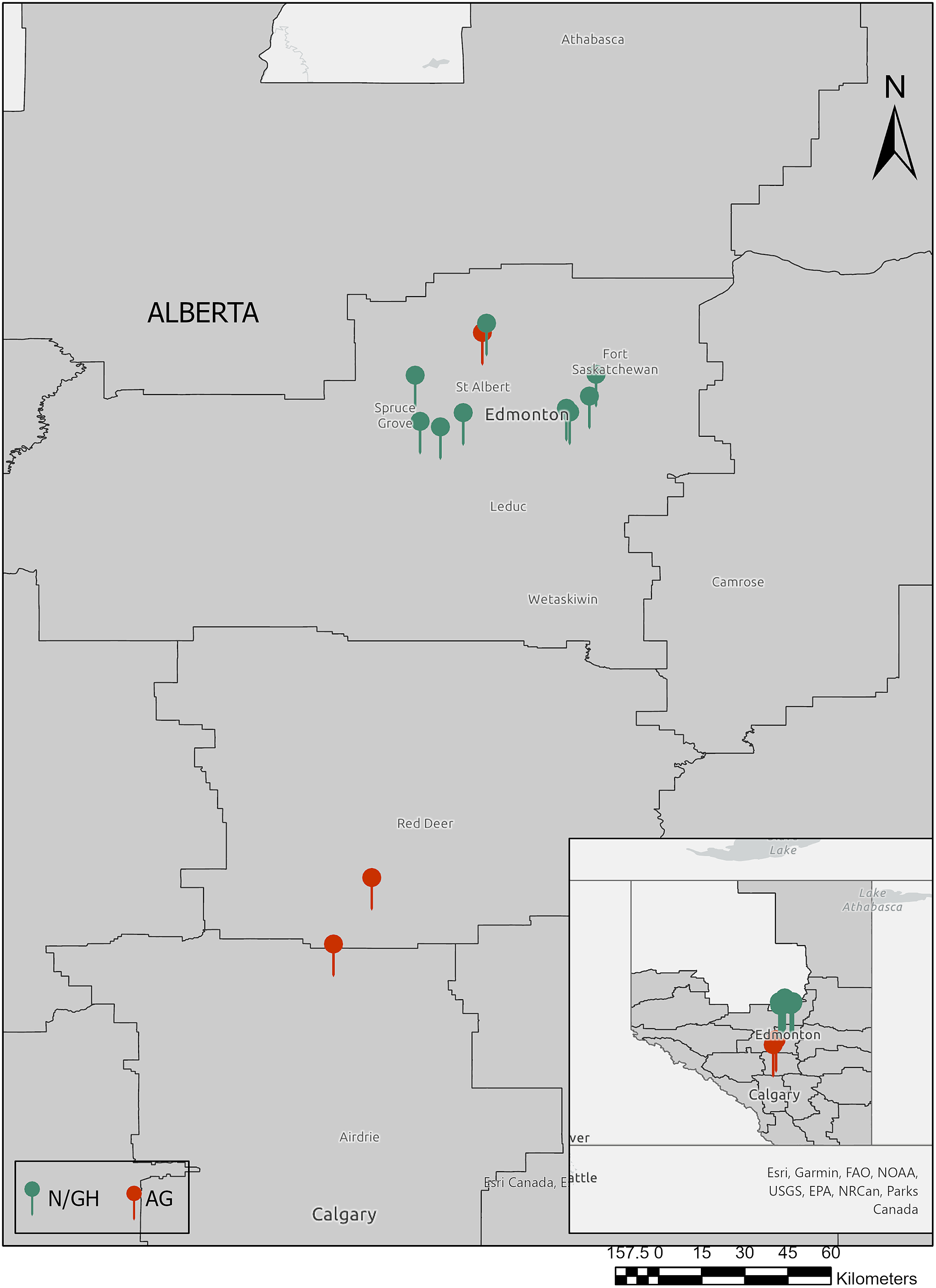
Figure 1. Slug survey (2021) locations. Slugs were collected from ten horticultural sites: i.e., nurseries and greenhouses (N/GH; green pins) and three agricultural sites (AG; red pins) in and around Edmonton, Alberta (main map). ArcGIS Pro software was used for visualization. (Esri, USGS | Sources: NRCan, Esri Canada, and Canadian Community Maps contributors. | Esri Canada | Northwest Territories, State of Alaska, Esri Canada, Esri, HERE, Garmin, FAO, NOAA, USGS, EPA, NPS, NRCan, Parks Canada
Slugs from residential gardens
In addition to the field-collected slugs, we occasionally received slugs from residential gardens in Alberta. Most of these samples were sterilized and used for slug colonies, except for 74 slugs (D. reticulatum) that were haphazardly chosen and placed on white traps to check for nematodes.
Nematode isolation
All slugs, except 55 (reserved for laboratory slug colonies) were rinsed thoroughly with distilled water to remove any external phoretic nematodes. The slugs were then decapitated to encourage the emergence of associated nematodes. The slug cadavers were kept in individual Petri dishes (6 cm diameter) lined with a damp paper towel, covered, and sealed with ParafilmTM. The cadavers were incubated at 18 °C, 80% relative humidity, 12 h light; 12 °C, 60% relative humidity, 12 h dark for two weeks. After this time, cadavers were inspected under a stereomicroscope. Nematodes were collected into 95% ethanol or DESS (dimethyl sulphoxide, disodium EDTA, and saturated NaCl) for subsequent molecular identification (Brophy et al. Reference Brophy, Mc Donnell, Howe, Denver, Ross and Luong2020b; Yoder et al. Reference Yoder, De Ley, King, Mundo-Ocampo, Mann, Blaxter, Poiras and De Ley2006).
Molecular identification
Slugs
A piece of the tail tip was clipped from select slug specimens (n = 24) and stored in 95% ethanol for molecular identification. QIAGEN DNeasy Blood and Tissue Kit (Germantown, MD, USA) was used to extract slug DNA from a ∼2 mm × 2 mm piece of slug tissue. Polymerase chain reaction cycling conditions followed Reich et al. (Reference Reich, Gormally, Allcock, Mc Donnell, Castillejo, Iglesias, Quinteiro and Smith2015). Partial fragments of the mitochondrial cytochrome c oxidase subunit I (cox 1) gene were sequenced: Cox 1 primer set LCO-1490 (5′-GGTCA ACAAATCATA AAGATATTGG-3′) and HCO-2198 (5′-TAA ACTTCAGGGTGACCAAAAAATCA-3′) (Folmer et al. Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994). Geneious Prime® software (North America Biomatters Inc, San Diego, CA, USA) was used to trim and edit the resulting sequences. The latter was then blasted on GenBank (Benson et al. Reference Benson, Cavanaugh, Clark, Karsch-Mizrachi, Lipman, Ostell and Sayers2013) to identify the species. In all cases, query coverage was >99.5%; the E value was 0; and the highest percentage identity was >99.0%. Sequences were deposited in GenBank under accession numbers OQ642082-OQ642105.
Nematodes
Nematodes were transferred from either 95% ethanol or DESS to a proteinase-K-based lysis buffer for DNA extraction (Williams et al. Reference Williams, Schrank, Huynh, Shownkeen and Waterston1992). Primer sets 18A (5′-AAAGATTAAGCCATGCATG-3′) and 26R (5′-CATT CTTGGCAAATGCTTTCG-3′) were used in PCR amplification and direct-end sequencing of the 18S ribosomal DNA, as previously described (Blaxter et al. Reference Blaxter, De Ley, Garey, Liu, Scheldeman, Vierstraete, Vanfleteren, Mackey, Dorris, Frisse and Vida1998; Denver et al. Reference Denver2003). The resulting 18S rRNA sequences were compared against GenBank’s non-redundant (nr) database using Standard Nucleotide BLAST (Basic Local Alignment Search Tool), (NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic AcidsRes.2018;46(D1):D8-D13. doi:10.1093/nar/gkx1095. DNA sequences generated for this study were submitted to GenBank under accession numbers OQ645705 - OQ645739 (Supplementary Table S1).
Results
A total of 1331 slugs were collected from agricultural sites, greenhouses, and commercial nurseries in Alberta from June to September 2021. These comprised nine species: Arion fasciatus, A. hortensis, A. rufus, A. subfuscus, Ambigolimax valentianus, Deroceras invadens, D. laeve, D. reticulatum, and Prophysaon andersonii. Of the 551 slugs collected from the three agricultural sites, we identified four species: A. fasciatus, D. invadens, D. laeve, and D. reticulatum (the most abundant, Table 1). We collected a total of 780 slugs from ten greenhouses and nurseries, representing all nine slug species. The most prevalent slug species encountered in these sites were D. laeve, A. valentianus, and D. reticulatum (Table 1). Slug abundance rose gradually over the summer, peaking in August (Figure 2).
Table 1. Abundance of the slug species collected in 2021 from Alberta agriculture sites, greenhouses, and nurseries. The following number of slugs were used to initiate laboratory slug colonies: Arion fasciatus (34), A. hortensis (4), A. rufus (3), A. subfuscus (3), Ambigolimax valentianus (3), Deroceras laeve (4), and Prophysaon andersonii (4).

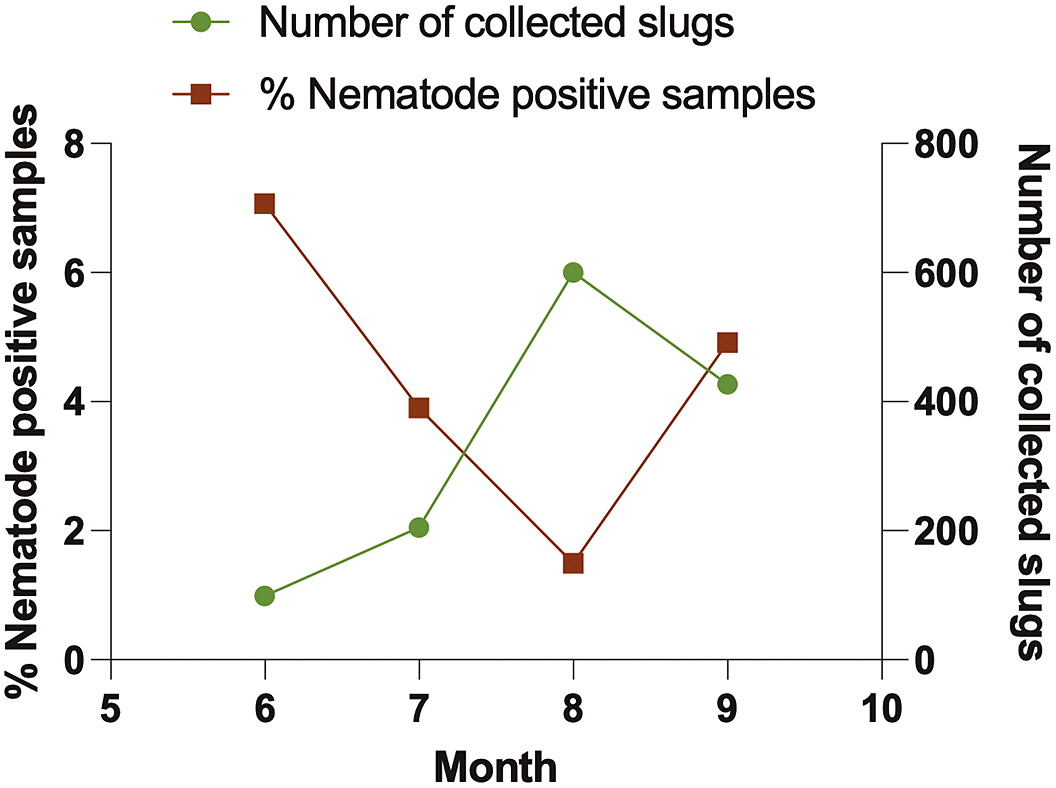
Figure 2. Temporal pattern of the total number of slugs collected from agriculture sites, nurseries/ greenhouses (green line), and nematodes isolated (%) from slug cadavers (brown line).
Out of 1331 slug samples collected during the survey, only 45 samples (3.38%) harbored nematodes (Table 2), but Phasmarhabditis was not isolated. The percentage of nematode-positive samples was high in June, followed by a gradual decline until August, but rose again in September (Figure 2). More nematode-positive samples were reported from nurseries and greenhouses (4.62%) than from agricultural (1.63%) sites (Table 2). Nematodes were isolated from four slug species: A. rufus, A. valentianus, D. laeve, and D. reticulatum, all of which were collected from two agricultural sites and four greenhouses/nurseries (Table 2). We identified 21 samples of nematodes to species level: Alloionema appendiculatum, Caenorhabditis briggsae, Caenorhabditis elegans, Panagrolaimus subelongatus, and Mesorhabditis spiculigera. Of all the nematode species, C. elegans and P. subelonatus were common to two agricultural sites and greenhouses/nurseries; the most prevalent nematode species was C. elegans (35.56%). By comparison, A. appendiculatum, M. spiculigera, and C. briggsae were only found in greenhouses and nurseries. Alloionema appendiculatum was isolated from a single A. rufus slug cadaver, while C. briggsae and M. spiculigera were isolated from an A. valentianus and a D. laeve slug cadaver respectively (Table 2).
Table 2. Slug-associated nematode species and their prevalence

AR, A. rufus; AV, A. valentianus; DR, D. reticulatum; DL, D. laeve
Of 74 slug cadavers, 22 samples (29.7%) from residential gardens were associated with nematodes. Of all positives, four samples were P. californica (18.18%). Other nematode species identified to the species level included C. elegans, Choriorhabditis cristata, Pristionchus entomophagus, and P. subelongatus. Other (n = 6) nematodes were only identified to the genus level: Panagrolaimus sp. and Rhabditophanes sp. (Table 2). The most common nematode species for both residential gardens and the survey sites were C. elegans and P. subelongatus, while C. cristata, P. californica, P. entomophagus, and Rhabditophanes sp. were only found in residential gardens.
Discussion
This study aimed to conduct a more extensive survey of agricultural and horticultural areas following Brophy et al. (Reference Brophy, Howe, Denver and Luong2020a; Reference Brophy, Mc Donnell, Howe, Denver, Ross and Luong2020b), including nurseries where the nematode P. californica was previously isolated from a single slug. In total, 1331 slugs were collected, with peak slug collection in August, similar to what was reported by Brophy et al. (Reference Brophy, Mc Donnell, Howe, Denver, Ross and Luong2020b). The slugs belonged to nine species from four families: Arionidae, Limacidae, Agriolimacidae, and Anadenidae. All slug species except P. andersonii (native) and D. laeve (exotic and native) are of European origin and were likely introduced to Canada via various means, e.g., trade and commerce of agricultural and horticultural commodities and/or travel (Araiza-Gómez et al. Reference Araiza-Gómez, Naranjo-García and Zúñiga2017; Grimm et al. Reference Grimm, Forsyth, Schueler and Karstad2009). Collectively, D. reticulatum was the most abundant slug species recorded during the survey (49.9%), the majority coming from agriculture sites (72.9%). The most common slug species found in greenhouses and nurseries were D. laeve (41.5%), A. valentianus (26.9%), and D. reticulatum (23.1%). Brophy et al. (Reference Brophy, Howe, Denver and Luong2020a; Reference Brophy, Mc Donnell, Howe, Denver, Ross and Luong2020b) collected 2406 slugs belonging to nine slug species from 82 sites including natural green spaces (e.g., parks and ravines), seasonal nurseries, and greenhouses, with the majority coming from residential gardens in and around Edmonton, Alberta. In comparison, we only collected about half the number of slugs during the current survey, likely due to lower slug emergence under elevated temperatures and unseasonably dry conditions during the survey period in 2021 compared to the year 2019 [i.e., 2019 July Olds college Agricultural Drought Monitoring Network (AGDM): maximum air temp ranged from 27.8°C–10.6°C, soil moisture at 005cm depth 39.4%–21%; 2021 July Olds college AGDM: maximum air temp ranged from 35.6°C–17.6°C, soil moisture at 005cm depth 19.1%–16.1%., Weather data provided by Agriculture and Irrigation, Alberta Climate Information Service (ACIS), https://acis.alberta.ca (February 2023)]. Further, unlike the previous survey by Brophy et al. (Reference Brophy, Howe, Denver and Luong2020a; Reference Brophy, Mc Donnell, Howe, Denver, Ross and Luong2020b), our survey did not focus on slugs from residential gardens. We did not collect Limax maximus, despite its recovery during the previous survey (n = 2) by Brophy (Reference Brophy, Howe, Denver and Luong2020a; Reference Brophy, Mc Donnell, Howe, Denver, Ross and Luong2020b). The failure to recover any would most likely be due to the extremely low population size of this slug species in and around the nursery where they were initially found.
Retail nurseries play a significant role as a focal point of terrestrial slug dispersal as they facilitate the passive transportation of exotic and native slugs with plant material to and from the nurseries (Bergey et al. Reference Bergey, Figueroa, Mather, Martin, Ray, Kurien, Westrop and Suriyawong2014; Schurkman et al. Reference Schurkman, Tandingan De Ley, Anesko, Paine, Mc Donnell and Dillman2022b). This could presumably cause the slugs to be transported over great distances, even provincial/ inter-state movements, with the primary destination being home gardens. Of the slug donations received from residential gardens, we identified two slug species, D. reticulatum and A. fasciatus. The latter was only received from a single residential garden and is the first report of A. fasciatus in a residential garden in Edmonton, Alberta. The dispersal of this slug species could have been a result of the horticulture trade (i.e., we collected A. fasciatus in greenhouses and nurseries in this study), with slugs being transported as adults, juveniles, and/or eggs residing in plant material and associated soil. Pest slug species including A. fasciatus and D. reticulatum are most likely to thrive in the absence of their natural enemies and are hard to eradicate once established (Kozlowski Reference Kozlowski2012; Robinson & Hollingsworth Reference Robinson and Hollingsworth2005). Their presence often results in persistent plant damage in home gardens and significant economic losses to large-scale crop farmers. Those may include yield loss and expenditures on labor, chemicals, and time required for the management of these pests. Further, these pest gastropods are ecologically important as they replace the detritivorous gastropod species that are mostly non-pests and native to the region, resulting in habitat alteration and reduced biodiversity (Kozlowski Reference Kozlowski2012). Therefore, we recommend regular monitoring and proper quarantine practices be in place, especially in retail nurseries.
In our study, D. reticulatum was the predominant slug species collected in agricultural sites. Besides D. reticulatum, only three others, A. fasciatus, D. invadens, and D. laeve were recovered but in fewer numbers. Douglas & Tooker (Reference Douglas and Tooker2012) listed D. reticulatum, D. laeve, A. subfuscus, and A. fasciatus as the most common slug species in field crops in the mid-Atlantic United States, and those species were not commonly associated with damage except D. reticulatum. In the Pacific Northwest region of the United States, D. reticulatum is thought to cause an estimated $60 million annual loss to the grass seed industry (Mc Donnell et al. Reference Mc Donnell, Colton, Howe and Denver2020). During the current study, D. laeve was reported primarily from wheat field margins in Innisfail and around 5cm deep in the soil in one of the post-harvest fields in St. Albert. A. fasciatus and D. invadens were recovered mainly from wheat field margins in Innisfail and canola field margins in Olds, respectively. They were mostly seen associated with marginal vegetation with no visible direct damage to the cash crops. However, the damage (active feeding resulting in irregular holes and tears in foliage) was obvious with D. reticulatum in the fields in Innisfail. Marginal vegetation strips appear to provide ideal conditions for slugs, where they retain moisture and are in close proximity to crop fields. This observation aligns with those by Frank (Reference Frank1998), who found that D. reticulatum occurred in large numbers in both grass strips and adjacent rape fields in Belp, Switzerland. Further, it was reported that the marginal rape plants were more vulnerable to slug damage (defoliation), especially by D. reticulatum. A global survey recently revealed that slugs, and Deroceras species in particular, are serious pests of rapeseed (Brassica napus) production (Zheng et al. Reference Zheng, Koopmann, Ulber and Von Tiedemann2020). Canada is one of the major rapeseed producers in the world, yet little is known about the extent of slug damage in Canada. This highlights the need for more systematic surveys to assess the abundance and distribution of pest gastropods and their economic impact on crops across the country. Slug damage is often identified by the presence of slime trails on crop plants or the soil; however, certain symptoms could be similar to those of other organisms such as wireworms (Keiser et al. Reference Keiser, Häberli and Stamp2012) and black cutworms (Chandel et al. Reference Chandel, Verma, Rana, Sanjta, Badiyala, Vashisth, Kumar and Baloda2022). This might result in the overestimation or underestimation of crop damage by pest slugs. Thus, we also recommend robust quantitative assessments of damage caused by slugs to a variety of crops grown under different conditions (e.g., till v no-till) using different management strategies.
Nematodes were recovered from four slug species (A. rufus, A. valentianus, D. reticulatum, and D. laeve) collected from agricultural sites, greenhouses, and nurseries. We isolated nematodes belonging to four families: Alloionematidae, Rhabditidae, Panagrolaimidae, and Neodiplogasteridae. Twenty-one samples of nematodes were identified to five species; the rest remained unidentified due to mixed populations of nematodes or due to impurities. Nurseries— which have a more frequent exchange of plant material than crop fields—are more likely to support a higher diversity of slug-associated nematodes than agriculture fields with a single crop or a few crops cultivated with a less frequent exchange of plants. This was evident in our study by the higher prevalence of slug-associated nematodes in greenhouses and nurseries (4.62%) compared to the agricultural fields (1.63%). In greenhouses and nurseries, the nematodes were isolated from A. rufus, A. valentianus, and D. laeve (Table 2). Of the slugs collected, A. rufus only harbored Alloionema appendiculatum, a known parasite of Arion spp., including Arion vulgaris, a serious pest in central Europe (Nermut et al. Reference Nermuť, Půža and Mráček2019). Despite A. appendiculatum causing high snail mortality in heliculture (Nermut et al. 2019), research has shown that this nematode had no success causing mortality in A. vulgaris under laboratory conditions, suggesting that this nematode has limited potential as a biocontrol agent against A. vulgaris (Nermut et al. Reference Nermuť, Půža and Mráček2019). Surprisingly, D. reticulatum, the third most abundant slug species in greenhouses and nurseries, had no associated nematodes. However, among the agriculture sites, D. reticulatum was the only slug species to harbor nematodes (1.86%). We did not recover P. californica from any of the slugs in the agricultural or horticultural sites we surveyed. However, P. californica (accession number KM510210) was isolated from half of the D. reticulatum slug cadavers (4/8) from one of the residential garden samples in Lethbridge, Alberta. This nematode may have a patchy distribution in the province, and as such more extensive surveys are needed to determine its true range. Previously, P. californica has been isolated from greenhouses and nurseries in different parts of North America (Schurkman et.al. Reference Schurkman, Tandingan De Ley, Anesko, Paine, Mc Donnell and Dillman2022b), including Alberta, Canada (Brophy et al. Reference Brophy, Howe, Denver and Luong2020a; Brophy et al. Reference Brophy, Mc Donnell, Howe, Denver, Ross and Luong2020b), but never from residential gardens. Therefore, this is the first report of P. californica being isolated from slugs in a residential garden in North America. Our results along with Brophy et al. (Reference Brophy, Howe, Denver and Luong2020a; Reference Brophy, Mc Donnell, Howe, Denver, Ross and Luong2020b) demonstrate that the Canadian strain of P. californica can utilize both D. reticulatum and A. rufus as its host. We further suggest the possibility that the abundance/ presence of P. californica community dynamics in 2021 compared to 2019 may be due to the impact of the climatic variables (unseasonably dry conditions in 2021) and/or nursery substrate origins. Some Phasmarhabditis species, including P. hermaphrodita, P. californica, and P. papillosa, have been shown to cause mortality in D. reticulatum (Mc Donnell et al. Reference Mc Donnell, Colton, Howe and Denver2020). Further, P. californica has a cosmopolitan distribution and has been isolated in Wales (Andrus & Rae Reference Andrus and Rae2019a; Andrus & Rae Reference Andrus and Rae2019b), Ireland (Carnaghi et al. Reference Carnaghi, Rae, De Ley, Johnston, Kindermann, Mc Donnell, O’Hanlon, Reich, Sheahan, Williams and Gormally2017), the USA (De Ley et al. Reference De Ley, Holovachov, Mc Donnell, Bert, Paine and De Ley2016; Mc Donnell et al. Reference Mc Donnell, Colton, Howe and Denver2020), New Zealand (Wilson et al. Reference Wilson, Wilson, Aalders and Tourna2016), and Canada (Brophy et al. Reference Brophy, Howe, Denver and Luong2020a; Brophy et al. Reference Brophy, Mc Donnell, Howe, Denver, Ross and Luong2020b). Given that D. reticulatum also has a global distribution and has been reported as a pest in Europe, North America, Australia, and New Zealand (Howlett Reference Howlett2012), there could be an opportunity to utilize P. californica as an alternative biocontrol agent against D. reticulatum in the absence of P. hermaphrodita. In fact, this species was commercialized (Nemaslug 2.0) for the UK market in 2022 (Mc Donnell et al. Reference Mc Donnell, Howe and Denver2023). However, different strains of P. californica have shown striking differences in host preference to slug cues (Cutler & Rae Reference Cutler and Rae2021). Therefore, we recommend further research on the host preference, infectivity, and pathogenicity of the Canadian strain of P. californica to determine its efficacy as a potential biocontrol agent of pest slugs.
Other than P. californica, Pristionchus entomophagus was also isolated from residential slug samples during the current study. Pristionchus species show a species-specific necromenic association with beetles (Brown et al. Reference Brown, D’Anna and Sommer2011). However, they can hitchhike on other insects to reach their final hosts. Interestingly, we observed a non-insect host interaction of Pristionchus sp. during this study, which we found in association with D. reticulatum (n = 4). This aligns with the observations by Brophy et al. (Reference Brophy, Mc Donnell, Howe, Denver, Ross and Luong2020b), who also found this taxon associated with D. reticulatum. This nematode is capable of producing dauer larvae under low food availability and infesting hosts (Brown et al. Reference Brown, D’Anna and Sommer2011). However, these necromenic nematodes cause no mortality to the host but wait until it dies to feed on the bacteria and resume development (Ishaq et al., Reference Ishaq, Hotopp, Silverbrand, Dumont, Michaud, MacRae, Stock and Groden2021; Sommer & McGaughran Reference Sommer and McGaughran2013). We suggest that the dauers we observed on slugs had a phoretic association, and when grown on D. reticulatum slug cadavers under laboratory conditions, P. entomophagus successfully propagated, suggesting that dead slugs are also a suitable growth substrate for this nematode taxon.
The introduction of a ‘potentially exotic’ nematode species as a biocontrol agent is restricted in many countries by legislation, and as such the importation and release regulations of those organisms are limited, often with the exception of those proven to be indigenous (Ehlers Reference Ehlers, Grewal, Ehlers and Shapiro-Ilan2005; Wilson et al. Reference Wilson, Wilson, Aalders and Tourna2016). Therefore, it is important to extend future survey efforts into the rest of Alberta, especially in and around the Lethbridge region, and into other provinces in Canada to verify the presence and distribution of Phasmarhabditis species. Further, there should be a thorough investigation of the infectivity and pathogenicity of this nematode on potential slug hosts to determine if this strain could be used as a biocontrol agent in Canada. Keyte et al. (Reference Keyte, Grannell, Sheehy, Shepherd and Rae2022) reported a higher prevalence of P. californica and P. hermaphrodita in snail hosts than in slugs. P. californica has been isolated from snail species such as Theba pisana (Tandingan De Ley et al. Reference Tandingan De Ley, Schurkman, Wilen and Dillman2020), Discus rotundatus (Keyte et al. Reference Keyte, Grannell, Sheehy, Shepherd and Rae2022), and Oxychilus draparnaudi in Germany and the UK (Grannell et al. Reference Grannell, Cutler and Rae2021; Keyte et al. Reference Keyte, Grannell, Sheehy, Shepherd and Rae2022). Therefore, future studies on nematodes associated with both terrestrial snails and slugs may reveal a wider distribution of Phasmarhabditis spp. in Canada. Relatively few studies have been conducted to determine the recent distribution and environmental impact of the introduced pest gastropods in Canada. There are even fewer studies on pest slugs in agriculture and horticulture habitats, which are of great economic importance. Here we report seven introduced and two native slug species in Alberta which are of agricultural and horticultural significance and the nematodes associated with those slugs. However, the economic damage and ecological impact of those slug species are poorly understood. P. californica has the potential to be used as a biological control agent in Canada due to its ability to cause mortality in pest slugs. Further studies including impacts on native non-target species are necessary to better understand the biological and ecological aspects of this nematode before an informed decision can be made on the use of this nematode as a slug control tool.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0022149X23000226.
Acknowledgements
We thank all the horticultural/agricultural facility staff for granting access. We appreciate the contribution of residential gardeners in providing slugs for colony maintenance. We are also grateful to T. Volappi and L. MacLeod for assistance in field collection and monitoring of slugs. Weather data provided by Agriculture and Irrigation, Alberta Climate Information Service (ACIS) https://acis.alberta.ca (February 2023).
Financial support
This project was funded by Alberta Environment and Parks.
Competing interest
RMcD declares that he is a co-inventor on a patent application entitled Mollusk-killing Biopesticide (U.S. application Serial No. 62/236,674).
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.






