Introduction
Digeneans of the family Fellodistomidae are generally restricted to the marine environment, with sexual adults (maritae) inhabiting fishes, and bivalves acting as first intermediate hosts (Bray & Gibson, Reference Bray and Gibson1980). Most fellodistomids are characterized by three- or two-host life cycles (Køie, Reference Køie1979, Reference Køie1980; Cribb et al., Reference Cribb, Bray, Olson and Littlewood2003), though with tendency to progenesis and lifecycle truncation up to one host (Stunkard & Uzmann, Reference Stunkard and Uzmann1959).
In the White Sea, the only broad-scale study of fish parasites was held more than 60 years ago (Shulman & Shulman-Albova, Reference Shulman and Shulman-Albova1953). Two fellodistomid species have been recorded in that study: Fellodistomum fellis (Olsson, 1868) in wolffish Anarhichas lupus Linnaeus, 1758 and Steringophorus furciger (Olsson, 1868) Odhner, 1905 in flatfishes (Pleuronectidae). Along with F. fellis, Fellodistomum agnotum Nicoll, 1909 is highly abundant in wolffish all over the North Atlantic and adjacent Arctic (Polyanski, Reference Polyanski1955; Bray & Gibson, Reference Bray and Gibson1980; Bray, Reference Bray1987). Fellodistomum fellis inhabits the gall bladder (younger specimens are occasionally found in the bile duct), and F. agnotum, the subglobular chamber at the base of the bile duct (Bray, Reference Bray1987). Fellodistomum agnotum exhibits morphological features intermediate between F. fellis and S. furciger (Bray & Gibson, Reference Bray and Gibson1980); thus, the taxonomic status and life cycles of these species have been questioned for a long time.
Among the three listed species, the complete life cycle was only elucidated for S. furciger through morphological and experimental data: protobranch bivalves Nuculana spp. serve as its only intermediate host (Chubrik, Reference Chubrik1966; Køie, Reference Køie1979). The life cycle of F. fellis with Ennucula tenuis (Montagu, 1808) as the first intermediate host and Ophiura sarsi Lütken, 1855 as the second intermediate host was described by Chubrik (Reference Chubrik1952, Reference Chubrik1966), but later it was rejected (Bray & Rollinson, Reference Bray and Rollinson1985). Metacercariae of F. fellis and F. agnotum were found in the stomach of a common whelk Buccinum undatum Linnaeus, 1758 (Bray, Reference Bray1987; Køie & Thulin, Reference Køie and Thulin1994). The first intermediate hosts of F. fellis and F. agnotum remain unknown, though they are suspected to be protobranch (Bray & Rollinson, Reference Bray and Rollinson1985) or mytilid (Køie & Thulin, Reference Køie and Thulin1994) bivalves.
In this paper, we record F. agnotum from the White Sea for the first time, provide evidence for the occurrence of F. agnotum and F. fellis metacercariae in buccinid gastropods, identify E. tenuis as the first intermediate host of F. agnotum and verify the life cycle of S. furciger by molecular analysis.
Material and methods
To obtain fellodistomid sexual adults, we collected wolffish and three species of flatfish (table 1) from the White Sea, Kandalaksha Bay, Keret Archipelago during summer 2019. They were dissected and the guts were examined under a stereomicroscope in the physiological solution. Fellodistomids obtained were identified and flat-fixed in 96% ethanol.
Table 1. Fellodistomid adults in wolffish and flatfishes.

For intensity, the range of values is followed by mean and standard error of mean in parenthesis.
Gastropods of the family Buccinidae – namely, B. undatum, Buccinum scalariforme Møller, 1842 and Neptunea despecta (Linnaeus, 1758) – were collected during summer–autumn 2019 at three different locations of the White Sea: around the Keret Archipelago, in Velikaya Salma Strait (both Kandalaksha Bay), and near Bolshoy Solovetsky Island (Onega Bay). During summer 2019, we collected all available species of protobranch bivalves at the first two locations: E. tenuis, Nuculana pernula (Müller, 1779), Portlandia arctica (Gray, 1824) and Yoldia hyperborea (Gould, 1841). Few E. tenuis and Y. hyperborea were collected in summer 2018 near Bolshoy Solovetsky Island. Molluscs were dissected in sea water and fellodistomid-like larvae were fixed in 96% ethanol. Photos of live worms were made with a Canon EOS 70D (Canon, Inc., Tokyo, Japan) camera and adaptor MFU (LOMO, Saint Petersburg, Russia) for the stereomicroscope MBS-10 (LOMO, Saint Petersburg, Russia).
For the morphological observations, worms were stained with Erlich's haematoxylin for 2–10 min, followed by destaining in 70% ethanol with 0.1 M hydrogen chloride for 1–12 h. Specimens were dehydrated through a series of graded alcohols and mounted in BioMount medium (Bio Optica, Milan, Italy). We photographed whole mounts with the compound microscope Leica DM 2500 (Leica Microsystems, Wetzlar, Germany ) and the camera Nikon DS Fi3 (Nikon, Tokyo, Japan). Measurements were made in Fiji software (Schindelin et al., Reference Schindelin, Arganda-Carreras and Frise2012).
Individual sporocysts, metacercariae and maritae were removed from 96% ethanol for DNA extraction (table 2). We then added 200 μl 5% Chelex-100 chelating resin (200–400-mesh particle size) (BioRad, Hercules, California, USA) and 2 μl proteinase K (20 mg/ml), incubated overnight at 56°C while mixing at 750 rpm (Eppendorf Comfort thermomixer; Eppendorf, Hamburg, Germany) and 8 min at 90°C. After centrifugation at 16,000 g for 10 min at −4°C, DNA was in supernatant. Polymerase chain reaction (PCR) amplification was carried out in 25 μl reaction mixtures, which contained 17 μl Milli-Q® water (Merck Millipore Co., Darmstadt, Germany), 5 μl ScreenMix-HS reaction mix (Evrogen, Moscow, Russia), 1 μl of each forward and reverse primers and 2 μl DNA template. Two primer pairs for 28S ribosomal DNA (LSU) variable regions were used: digl2, AAGCATATCACTAAGCGG (Tkach et al., Reference Tkach, Grabda-Kazubska, Pawlowski and Swiderski1999) and 1500R, GCTATCCTGAGGGAAACTTCG (Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003) for ~1200 base pairs (bp) D1–D3 domains; C2′B, GAAAAGTACTTTGRARAGAGA (Bayssade-Dufour et al., Reference Bayssade-Dufour, Jouet, Rudolfova, Horák and Ferté2000) and D2, TCCGTGTTTCAAGACGGG (Vân Le et al., Reference Vân Le, Lecointre and Perasso1993) for a ~600 bp D2 domain. The PCR thermal profile included initial denaturation at 95°C for 5 min; amplification cycles (35 for D2 or 40 for D1–D3) with 30 s at 95°C, 30 s at 53°C (D2) or 54°C (D1–D3), and 1 min (D2) or 2 min (D1–D3) at 72°C; and final elongation at 72°C for 10 min. PCR products were visualized with SYBR Green (Invitrogen, Carlsbad, California, USA) following electrophoresis in 1% agarose gel. PCR products were sequenced by automated Sanger method using an ABI Prism 3500xl (Applied Biosystems, Foster City, California, USA) with PCR primers. We evaluated chromatogram quality, assembled sequences from forward and reverse reads and aligned all sequences in Geneious Prime® 2019.2.1 (www.geneious.com). Species identification was confirmed by matching our sequences to those available in GenBank for F. agnotum, F. fellis and S. furciger from the North Sea (AJ405289, AJ405290 and AJ405292; Bray et al., Reference Bray, Littlewood, Herniou, Williams and Henderson1999). To estimate sequence divergence, we built an illustrative alignment covering all combinations of studied species and lifecycle stages, as well as GenBank voucher samples. To compute pairwise distances and standard error estimates (1000 bootstrap replicates) for this alignment, we used a maximum composite likelihood model in MEGA version 7 (Kumar et al., Reference Kumar, Stecher and Tamura2016); all positions containing gaps and missing data were eliminated. The accession numbers for the newly generated sequences are MT216732–MT216758 (table 2).
Table 2. Isolates, their origin and GenBank accession numbers for 28S rDNA sequences.
Results
Two species of the genus Fellodistomum – F. fellis and F. agnotum – were found in wolffish (16 examined). Fellodistomum fellis was found in the gall bladder at a prevalence of 87.5% and an intensity of 1–67 (14.9 ± 4.2). Fellodistomum agnotum was found in the subglobular chamber at the base of the bile duct at a prevalence of 50% and an intensity of 1–10 (4.6 ± 0.8) (table 1). Among three studied flatfish species, S. furciger maritae were present only in Limanda limanda at a prevalence of 16.7% and an intensity of 1–10 (4.8 ± 0.6). Worms were found mostly in the intestine, once in the stomach (two specimens) and once in the rectum (two specimens).
Two fellodistomids were found in bivalves (table 3). Sporocysts and cercariae similar to those previously described for S. furciger (Chubrik, Reference Chubrik1966; Køie, Reference Køie1979) were recovered from two N. pernula collected near Keret Archipelago. A single infected specimen of E. tenuis collected near Bolshoy Solovetsky Island contained sporocysts and large furcocercous cercariae, which were similar to ones described by Chubrik (Reference Chubrik1952, Reference Chubrik1966) as F. fellis, though an important difference was that in these cercariae the excretory vesicle was Y-shaped, not V-shaped (fig. 1). None of the Y. hyperborea (n = 57) and P. arctica (n = 8) were infected.

Fig. 1. Fellodistomum agnotum cercaria from Ennucula tenuis.
Table 3. Fellodistomid sporocysts and cercariae in protobranch bivalves.
Fellodistomid metacercariae were recovered from the digestive tract of B. undatum, B. scalariforme and N. despecta. All of the metacercariae could be clearly assigned to either F. fellis or F. agnotum based on morphology. The most prominent difference was in the excretory vesicle, which was V-shaped in F. fellis and Y-shaped in F. agnotum. It was most clearly visible in live worms (fig. 2), easily seen in fixed ones, but became scarcely discernible in mounted and stained specimens because they were too transparent (fig. 3). The size of ventral sucker (relative to oral sucker and body length) may be a good differential character (larger in F. fellis), but does not work so well for younger specimens when allometric growth is not yet prominent. The position of reproductive organs primordia differed in mature metacercariae of F. fellis and F. agnotum, but was also similar in younger specimens.
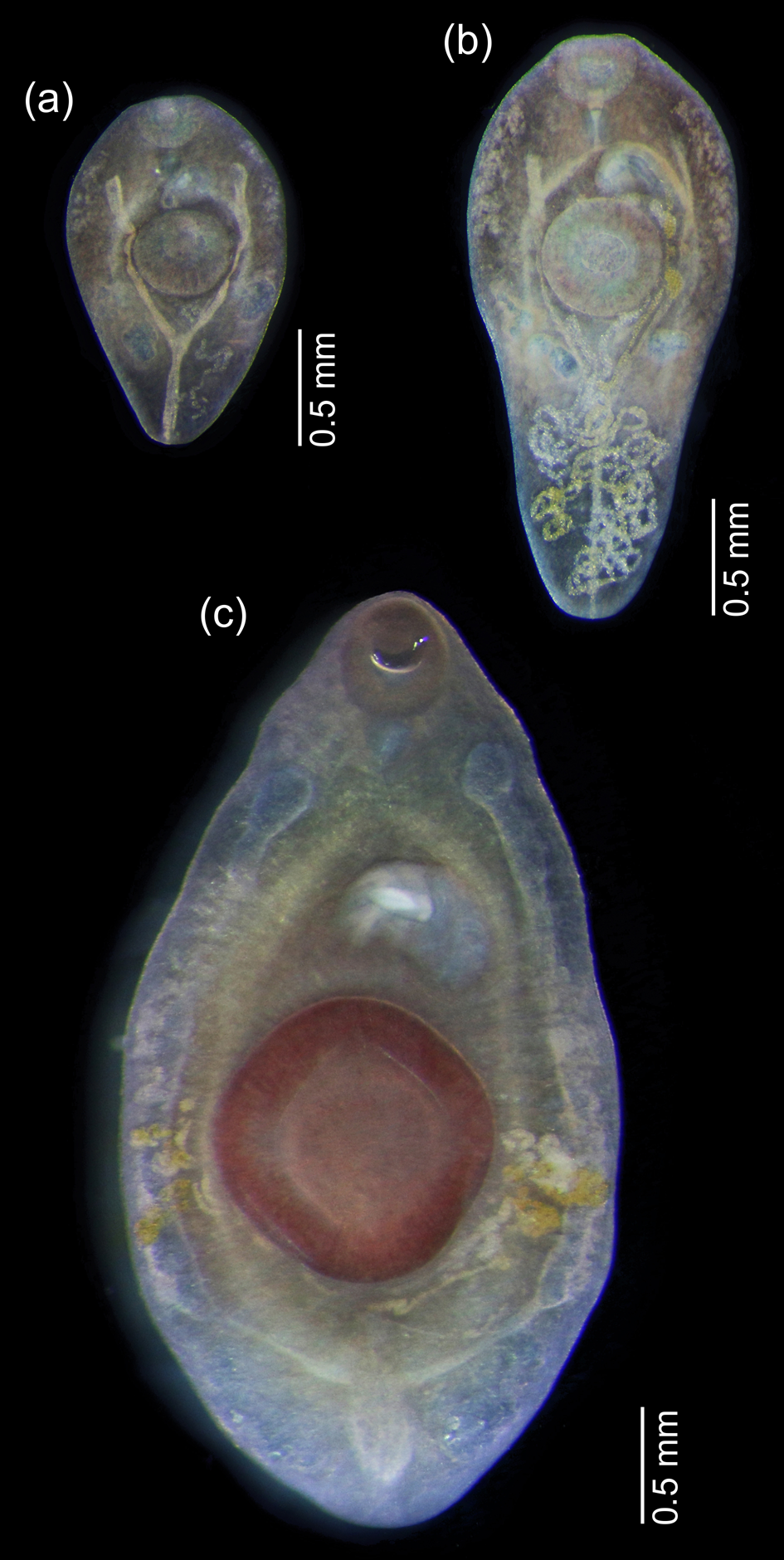
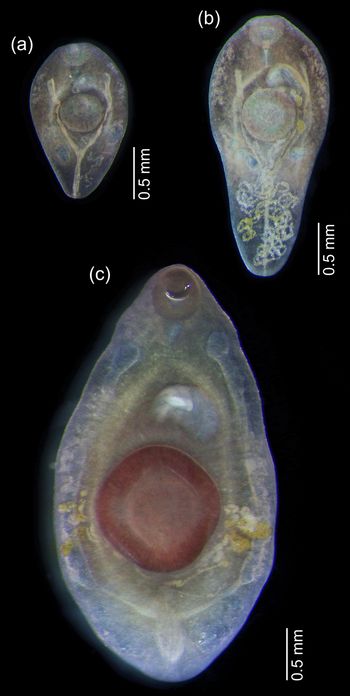
Fig. 2. Live metacercariae from Buccinum undatum: (a, b) Fellodistomum agnotum, note eggs in uterus; (c) F. fellis, the largest specimen. All worms are shown at the same scale.
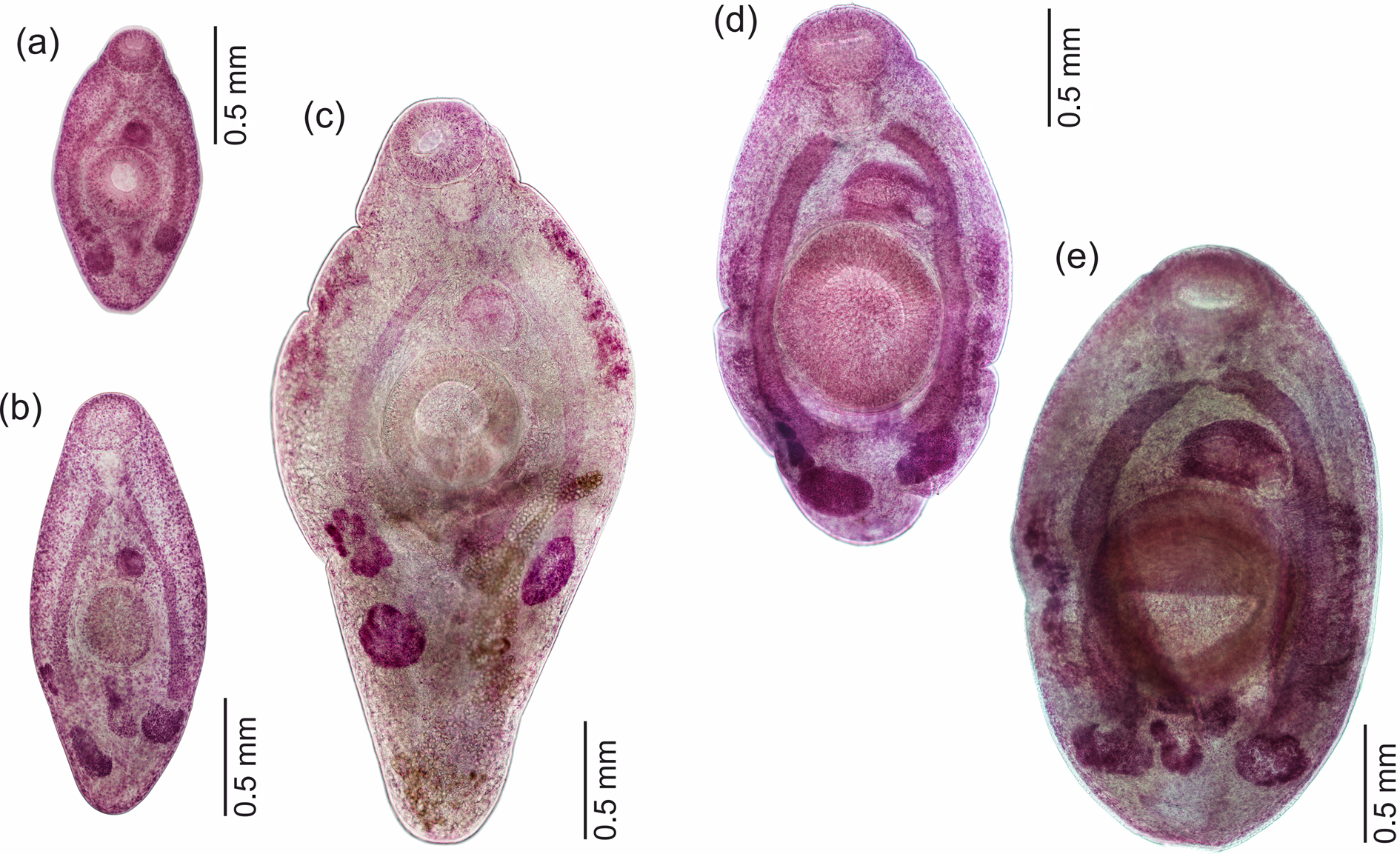
Fig. 3. Whole-mounted metacercariae of Fellodistomum agnotum (a–c) and F. fellis (d, e). Note eggs on (c). All worms are shown at the same scale.
Three of the seven F. agnotum metacercariae contained eggs in the uterus, and two of them did not differ from full-grown adults from A. lupus; the largest specimen was 3.2 mm long (figs 2 and 3). The largest F. fellis metacercaria was 4.0 mm long (fig. 2). Several F. fellis metacercariae had fully formed reproductive systems and had started producing a few eggs.
The majority of metacercariae were found in the stomach and oesophagus, with a few in the beginning of the intestine (table 4). For F. agnotum the intensity was 1, for F. fellis it was 1–3. A double infection was found thrice.
Table 4. Metacercariae of Fellodistomum agnotum and F. fellis in buccinid gastropods.
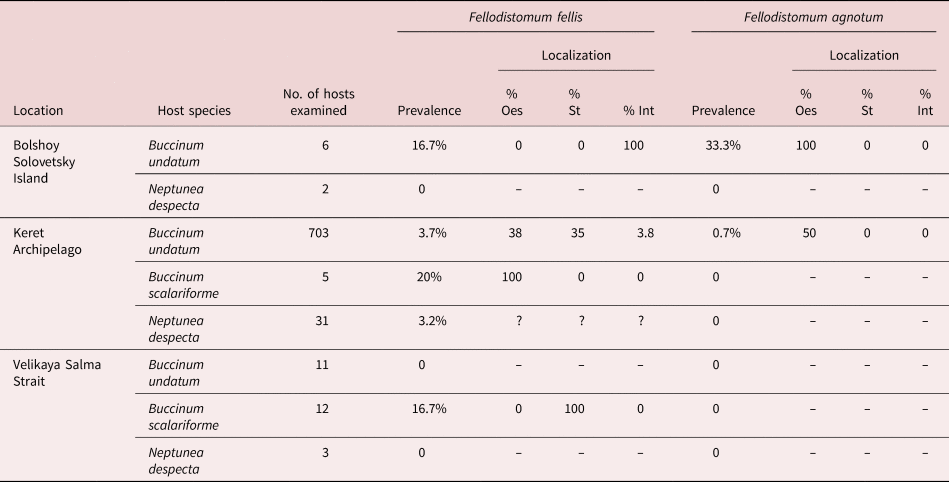
Localization shows % of worms in oesophagus (Oes), stomach (St) and intestine (Int); % sum may be not equal to 100 as we do not have localization data for some of the samples.
We obtained partial LSU sequences for the sporocyst from E. tenuis and for 24 Fellodistomum specimens, 11 maritae and 13 metacercariae, originating from 20 different host individuals (table 2). After trimming and quality control, six sequences were 1237–1257 bp long, corresponding to the D1–D3 variable LSU domains. Eight sequences were 546 bp long (D2 LSU) and 13 were 757–866 bp long (D1–D2 LSU). We also obtained two D1–D3 LSU sequences for S. furciger (sporocyst and marita).
All sequences from metacercariae and maritae of F. fellis (including AJ405290) were identical. The same was true for sequences from metacercariae and maritae of F. agnotum (including AJ405289) and sporocyst from E. tenuis, except for two ambiguous positions in MT216758. All F. fellis and F. agnotum differed consistently by three nucleotide substitutions restricted to the D2 LSU. When comparing six available longer (D1–D3) sequences – two for F. agnotum and four for F. fellis – one more segregating site was detected in position 956 of the 1257-bp-long alignment. Finally, there were also no differences between S. furciger sequences from sporocyst and maritae (including AJ405292). The alignment used to illustrate genetic distances within and between studied species included ten sequences and, trimmed to the shortest sequence, was 848 bp long (table 5).
Table 5. 28S rDNA sequence divergence between isolates of three fellodistomid species, featuring a subset of different developmental stages and host species.
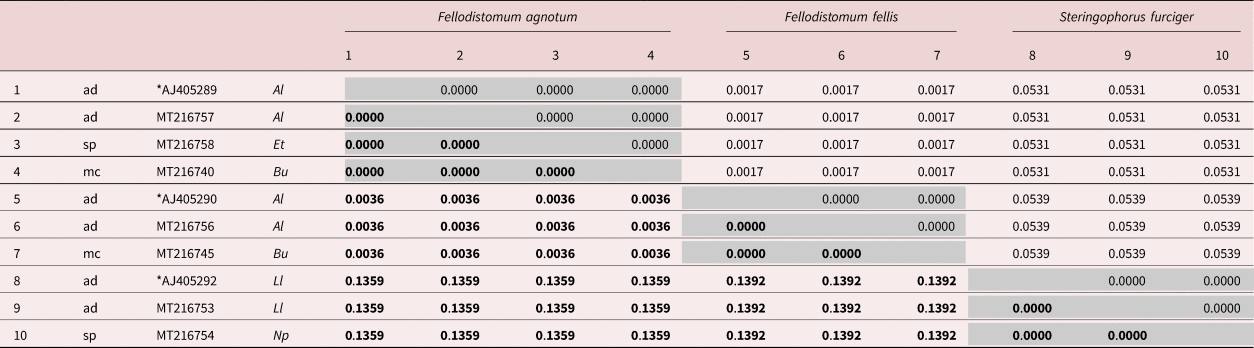
Developmental stages: ad, sexual adult; sp, sporocyst; mc, metacercaria. Host species: Al, Anarhichas lupus; Et, Ennucula tenuis; Bu, Buccinum undatum; Ll, Limanda limanda; Np, Nuculana pernula. Asterisks mark sequences from Bray et al. (Reference Bray, Littlewood, Herniou, Williams and Henderson1999) (isolates from the North Sea). All other sequences were generated in this study (isolates from the White Sea). Base substitutions per site are given in bold below the diagonal; Standard error values are given above the diagonal.
Discussion
Adult worms of F. agnotum were recorded in the White Sea for the first time basing both on morphological and molecular characteristics. We suggest that previous data on S. furciger from A. lupus (Shulman & Shulman-Albova, Reference Shulman and Shulman-Albova1953) may actually refer to F. agnotum. Bray (Reference Bray1987) discovered the same mistake in the records on the Barents Sea wolffish by Polyanski (Reference Polyanski1955).
The gastropods B. undatum, B. scalariforme and N. despecta were proved to be the second intermediate hosts for F. fellis, and B. undatum for F. agnotum, based on morphology and molecules, which complies with previous observations (Bray, Reference Bray1987). Anarhichas lupus probably gets infected by eating whelks, its common prey (Templeman, Reference Templeman1985; Bray, Reference Bray1987). We routinely found buccinid shells in the digestive tract of the White Sea wolffish.
Køie (Reference Køie1969) had observed F. agnotum with immature eggs in whelks (she described it as S. furciger). We also found metacercariae of F. agnotum with eggs three times, and two of them were as developed as full-grown adults from the definitive host. Several F. fellis metacercariae also produced eggs, but not many. It seems that F. agnotum is more predisposed to progenesis than F. fellis. As for the other fellodistomids, egg production in the intermediate host had been previously reported in Steringotrema ovacutum (Lebour, 1908) Yamaguti, 1954 by Køie (Reference Køie1980), though that was just three larger metacercaria specimens with few eggs. Also, progenetic development is very typical for two Proctoeces species (Stunkard & Uzmann, Reference Stunkard and Uzmann1959; Oliva & Huaquin, Reference Oliva and Huaquin2000). Thus, egg production by metacercariae appears to be frequent among Fellodistomidae. Viability and infectivity of eggs from Fellodistomum metacercariae is a matter for further studies.
Our LSU sequences of sporocysts from N. pernula matched those of S. furciger adults from L. limanda, as well as data from GenBank on this species. Thus, the two-host life cycle of S. furciger proposed by Chubrik (Reference Chubrik1966) and Køie (Reference Køie1979) was verified.
Sporocysts and furcocercous cercariae that we found in E. tenuis near Bolshoy Solovetsky Island were shown to belong to F. agnotum by evidence from LSU sequences. Thus, the life cycle of this species was resolved (fig. 4). Chubrik (Reference Chubrik1952, Reference Chubrik1966) reported large furcocercous cercariae from E. tenuis and considered them to be the larvae of F. fellis. Køie (Reference Køie1980) doubted this assumption and, according to her own results of experimental infection, decided that F. fellis is a synonym of S. ovacutum. This was rejected by enzyme electrophoresis analysis by Bray & Rollinson (Reference Bray and Rollinson1985), who attributed lifecycle data of Chubrik and Køie to S. ovacutum but not F. fellis. They also suggested that Cercaria megalocerca Chubrik, 1966 from bivalves P. arctica may be the larva of F. fellis. However, this is not likely as P. arctica is a high-arctic species and its distribution does not correspond to that of F. fellis (Warén, Reference Warén1989). We suspect that the first intermediate host of F. fellis may be E. tenuis, just like for F. agnotum, and the morphological differences between these species are inconspicuous at the cercaria and sporocyst stages. In this case, F. fellis and F. agnotum would have completely identical life histories except for the site of infection in the definitive host. Unfortunately, low accessibility of E. tenuis in the research areas confines our sample sizes, and we have not found a F. fellis infection yet.
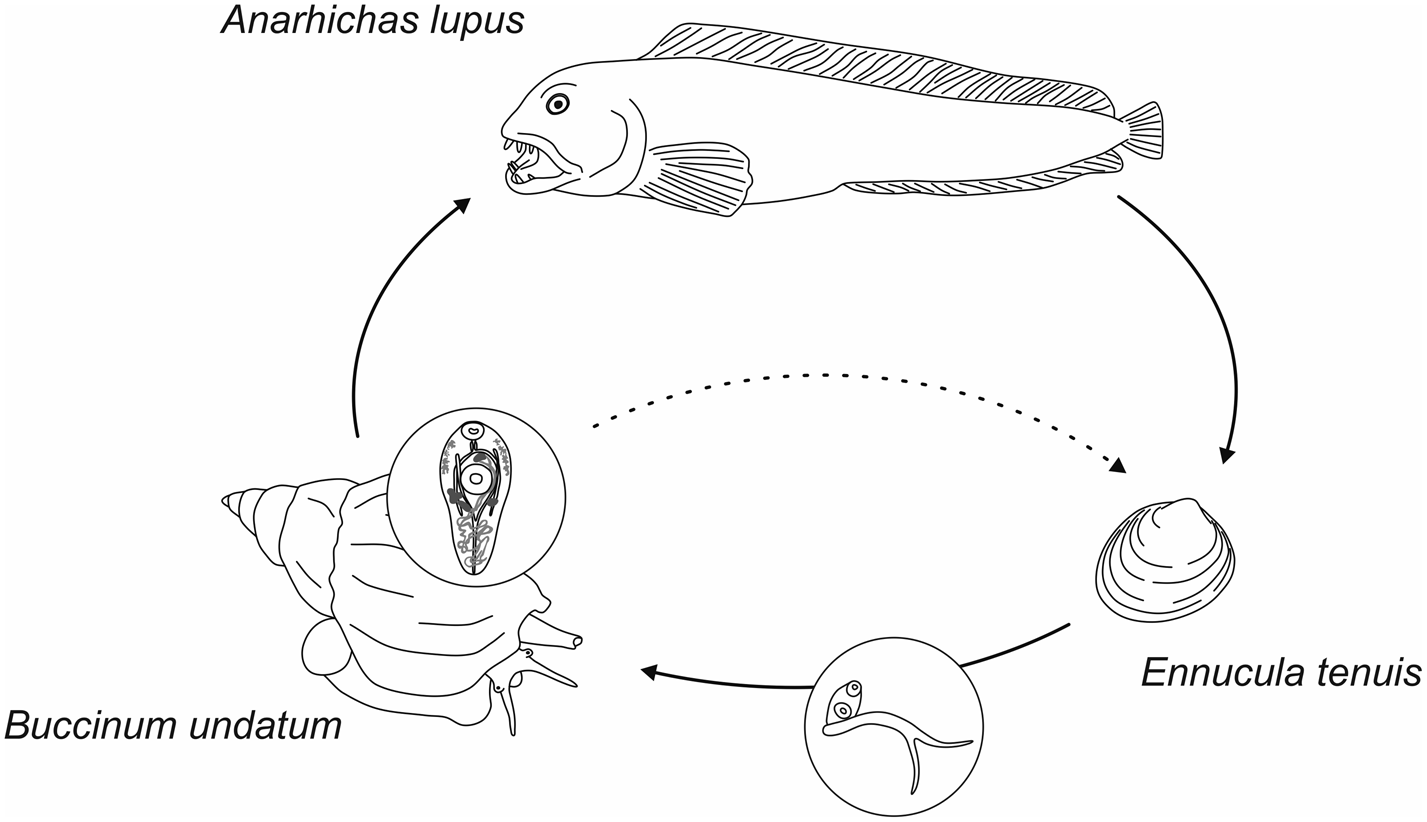
Fig. 4. Life cycle of Fellodistomum agnotum. Sporocysts and cercariae develop in protobranch bivalve Ennucula tenuis. Metacercariae inhabit digestive tract of whelk Buccinum undatum. Wolffish probably gets infected through eating whelks. Metacercariae may produce eggs within the second intermediate host, which makes lifecycle truncation possible.
The close relationship between F. fellis and F. agnotum is highlighted by the small genetic distance in the LSU fragment (0.36%; table 5). Among fellodistomids, a similar distance in LSU was observed between the supposed intraspecific groups of Proctoeces sicyases (Oliva et al., Reference Oliva, Valdivia, Cárdenas, Muñoz, Escribano and George-Nascimento2018) and between possible cryptic species within Proctoeces maculatus (Antar & Gargouri, Reference Antar and Gargouri2016; Wee et al., Reference Wee, Cribb, Bray and Cutmore2017). The interpretation of such similarity requires additional data (several genetic markers, lifecycle data, geographic range, etc.). The distinctness of F. fellis and F. agnotum is supported by evidence of various natures: morphology and location within the definitive host (Bray & Gibson, Reference Bray and Gibson1980), enzyme electrophoresis (Bray & Rollinson, Reference Bray and Rollinson1985), 18S rDNA (small subunit) (Lumb et al., Reference Lumb, Bray and Rollinson1993) and LSU (Bray et al., Reference Bray, Littlewood, Herniou, Williams and Henderson1999) sequences.
Conclusion
To sum up, our study has contributed to clarifying the life cycles of three species of White Sea fellodistomids: S. furciger, F. agnotum and (except for the first intermediate host) F. fellis. Many of the previous accounts on the life cycles of these species were controversial. Here, for the first time, we provided DNA sequence data for material from intermediate hosts, which served to ultimately resolve some of the disputes. However, many questions remain. One of them concerns other possible fellodistomid species from those White Sea fishes that have not yet been thoroughly surveyed. Another issue is the identity of fellodistomid cercariae from P. arctica (Chubrik, Reference Chubrik1966). Further study of the nature of divergence between two very close species, F. fellis and F. agnotum, is also worth attention.
Acknowledgements
The authors thank the Educational and Research Station ‘Belomorskaia’ of Saint Petersburg University (SPbU), the White Sea Biological Station ‘Kartesh’ of the Zoological Institute of the Russian Academy of Sciences (ZIN RAS) and the White Sea Biological Station of Lomonosov Moscow State University for providing fieldwork resources. Sampling at WSBS ‘Kartesh’ was supported by a ZIN RAS research program (project number AAAA-A19-119020690109-2). The molecular studies were carried out using the equipment belonging to the research resource centre ‘Molecular and Cell Technologies’ of SPbU.
Financial support
This study was supported by the Russian Science Foundation (project number 19-74-10029).
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.
Conflicts of interest
None.