Introduction
The genus Mylonchulus was proposed by Cobb (Reference Cobb1916) when he divided the genus Mononchus Bastian, 1865 into five genera, namely: Mononchus Bastian, 1865, Prionchulus Cobb, Reference Cobb1916, Mylonchulus Cobb, Reference Cobb1916, Iotonchus Cobb, Reference Cobb1916, and Anatonchus Cobb, Reference Cobb1916 and the species Mylonchulus minor (Cobb, 1893) Cobb, Reference Cobb1916 became the type species. Until 1976, the genus Mylonchulus Cobb, Reference Cobb1916 contained a small number of monodelphic species, and in that year, Andrássy transferred the only opisthodelphic species M. reversus Cobb, Reference Cobb1917 to the newly erected genus Oligonchus Andrássy, Reference Andrássy1976. In 1982, Jairajpuri and Khan proposed the new genus Paramylonchulus Jairajpuri and Khan, 1982, which was split from the genus Mylonchulus Cobb, Reference Cobb1916 based on some main characteristics such as: female genital system mono-prodelphic and the absence of teeth in subventral wall. Seven species including M. caespitosus Razzhivin, 1971, M. californicus Jairajpuri, Reference Jairajpuri1970, M. index Cobb, 1906, M. mashhoodi Khan and Jairajpuri, Reference Khan and Jairajpuri1979, M. mulveyi Jairajpuri, Reference Jairajpuri1970, M. silvaticus Razzhivin, 1971, M. subterraneus Schneider, 1940 were transferred to the new genus Paramylonchulus Jairajpuri and Khan, 1982 with P. index Cobb, 1906 as the type species. Khan and Saeed (Reference Khan and Saeed1987) proposed the new genus Pakmylonchulus Khan and Saeed, Reference Khan and Saeed1987 which differed from the genus Mylonchulus Cobb, Reference Cobb1916 by the absence of subventral teeth and the body length of less than 1 mm based on the type species of P. amurus Khan and Jairajpuri, Reference Khan and Jairajpuri1979. Loof (Reference Loof1993) did not accept that the number of female genital branches (didelphic or mono-prodelphic) is basis for generic separation between Mylonchulus Cobb, Reference Cobb1916 and Paramylonchulus Jairajpuri and Khan, 1982 and considered the Paramylonchulus Jairajpuri and Khan, 1982 as a synonym of the genus Mylonchulus Cobb, Reference Cobb1916. Zullini and Peneva (Reference Zullini, Peneva, Abebe, Andrassy and Traunspurger2006) and Andrássy (Reference Andrássy2009) agreed with Loof (Reference Loof1993) and did not accept the genera Paramylonchulus Jairapuri and Khan, 1982 and Pakmylonchulus Khan and Saeed, Reference Khan and Saeed1987 as valid genera only based on the characteristics of female genital system and the presence of teeth on subventral wall. These authors believed that those characteristics did not clearly differentiate these genera from Mylonchulus. Siddiqi (Reference Siddiqi2015) proposed the new genus Montonchus Siddiqi, Reference Siddiqi2015 which differed from the genus Mylonchulus Cobb, Reference Cobb1916 by having two widely spaced distinct ridges across the middle of the buccal cavity. Only one species Mylonchulus montanus (Thorne, 1924) Goodey, Reference Goodey1951 was transferred to the genus Montonchus Siddiqi, Reference Siddiqi2015 and became the type species. Aliramaji et al. (Reference Aliramaji, Taheri and Shokoohi2023) reconfirmed the validity of the genus Paramylonchulus based on analysis of morphological characteristics and molecular data of the species P. iranicus Aliramaji et al., Reference Aliramaji, Taheri and Shokoohi2023 from Iran. The main diagnostic features of the genus Mylonchulus are: (1) buccal cavity goblet or funnel-shaped, thickened walls; (2) dorsal tooth is very large, massive, claw-like, obliquely directed anteriad with sharply pointed apex and situated in the anterior half of buccal cavity; (3) submedian rasp-like denticles arranged regular transverse rows; (4) non-tuberculate cardia, (5) female reproductive system amphidelphic and (6) tail of both sexes similar, short (c´≤ 4), varying in forms; caudal glands well developed, grouped or in tandem; spinneret opening at the tail terminus (Ahmad and Jairajpuri Reference Ahmad and Jairajpuri2010).
Currently, 69 valid Mylonchulus Cobb, Reference Cobb1916 species have been described, with the three most recent species being M. kermaniensis Shokoohi et al., Reference Shokoohi, Mehrabi-Nasab, Mirzaei and Peneva2013 from Iran, M. capsicumi Ishaque et al., Reference Ishaque, Iqbal, Kazi, Dawar and Raza2021 from Pakistan and M. shamimi Handoo et al., Reference Handoo, Kantor and Khan2021 from India. In Vietnam, 20 species of the genus Mylonchulus have been reported (Vu Reference Vu2016).
In the present study, Mylonchulus laocaiensis sp. n. is described based on morphological, morphometric, and molecular data from Lao Cai Province, Vietnam.
Materials and methods
Nematode extraction, preservation and morphological studies
Soil samples were collected from a pristine forest in Bat Xat Nature Reserve and Hoang Lien National Park in Lao Cai Province, Vietnam. Nematodes were extracted from soil samples using the Baermann funnel technique (Southey, Reference Southey1986).
Specimens were heat killed at 70° fixed in TAF solution (Southey, Reference Southey1986) (for morphological observations) or in a DESS mixture (Yoder et al., Reference Yoder, De Ley, King, Mundo-Ocampo, Mann, Blaxter, Poiras and De Ley2006) (for molecular analyses), transferred to anhydrous glycerol (Seinhorst, Reference Seinhorst1962), and mounted on glass slides for microscopic observation. After filming and taking pictures, selected specimens were submitted for molecular studies. Measurements were performed with a Nikon digital camera on a Nikon Eclipse Ni microscope at the Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology (VAST), Vietnam. Observations of morphological diagnostic features and photographs were taken with a Nikon digital camera mounted on a Nikon Eclipse Ni microscope. Illustrations were drawn using a Nikon Eclipse Ni microscope equipped with a Nikon Y-IDT drawing tube. Photographs and illustrations were edited using Adobe Photoshop CC 2018.
DNA extraction, polymerase chain reaction and sequencing
Nematode DNA was extracted from a single individual as described by Holtermann et al. (Reference Holterman, Rybarczyk, Evan den Elsen, van Megen, Mooyman, Peña Santiago, Bongers and Helder2008), and DNA extracts were stored at –20° until used as a polymerase chain reaction (PCR) template. The D2-D3 expansion segment of 28S rDNA was amplified using the forward D2A (5′-ACAAGTACCGTGGGGAAAGTTG-3′) and reverse D3B (5′-TCGG AAGGAACCAGCTACTA-3′) primers (Subbotin et al., Reference Subbotin, Sturhan, Chizhov, Vovlas and Baldwin2006) and the 18S rRNA gene was amplified using primers NEM18SF: 5′-CGCGAATRGCTCATTACAACAGC-3′ (forward) and NEM18SR: 5′-GGGCGGTATCTGATCGCC-3′ (reverse) (Floyd et al., Reference Floyd, Rogers, PJD and Smith2005). All PCR reactions contained 12.5 μL Hot start green PCR Master Mix (2×) (Promega, USA), 1 μL of the forward and reverse primer (10 μM each), the 3 μL DNA template and sterile Milli-Q water to 25 μL of the total volume. All PCR reactions were performed in SimpliAmp Thermal cycler (Thermo Fisher Scientific) as follows: an initial denaturation step at 95° C for 4 min, followed by 40 cycles at 95° C for 30 s, 54° C for 30 s and 72° C for 60 s with a final incubation for 5 min at 72 °C. Amplicons were visualised under ultraviolet illumination after Simplisafe gel staining and gel electrophoresis. After sequencing the obtained Mylonchulus laocaiensis sp. n. rDNA sequence fragments were deposited in GenBank under the following accession numbers: MW54445, MW544446 (18S rDNA) and MW544448, MW544450 (D2D3 28S rDNA).
Phylogenetic analyses
Phylogenetic relationship analyses were based on 18S and 28S rDNA. The newly obtained rDNA sequences were analysed using the BioEdit sequences available in GenBank using the ClustalW alignment tool implemented in the MrBayes version 3.2.6 (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). The final 18S and 28S rDNA datasets for phylogenetic study included sequences from the current study Mylonchulus laocaiensis sp. n. and available sequences of Mononchida representatives from GenBank. The prepared multiple alignments of 18S and 28S rDNA generated by the ClustalW algorithm were routinely manually edited to eliminate improper phylogenetic signals. A 18S alignment of 885-bp long containing 48 sequences and an D2-D3 alignment of 800-bp long containing 45 sequences were created. Bayesian inference (BI) analysis was constructed with the program MrBayes version 3.2.6 (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). The analysis under the generalized time reversible and invariant sites and gamma distribution (GTR + I + G) model was initiated with a random starting tree and run with the Markov chain Monte Carlo (Larget & Simon, Reference Larget and Simon1999) for 1 × 106 generations. The tree was visualised and saved with FigTree 1.4.4 (Rambaut, Reference Rambaut2018).
Results and discussion
Description
Order: MONONCHIDA Jairajpuri, Reference Jairajpuri1969
Family: Mylonchulidae Jairajpuri, Reference Jairajpuri1969
Genus: Mylonchulus Cobb, Reference Cobb1916
Mylonchulus laocaiensis sp. n. (Figures 1, 2 and 3).
urn:lsid:zoobank.org:pub:3826F1CD-C115-4B89-9988-840C4C667B6A
Type material:

Figure 1. Holotype female and paratype male of Mylonchulus laocaiensis sp. n. (a): female head region; (b): male head region; (c): male tail region; (d): pharyngo-intestinal junction region; (e): female tail region; (f): female reproductive system (scale bars = 20 μm).
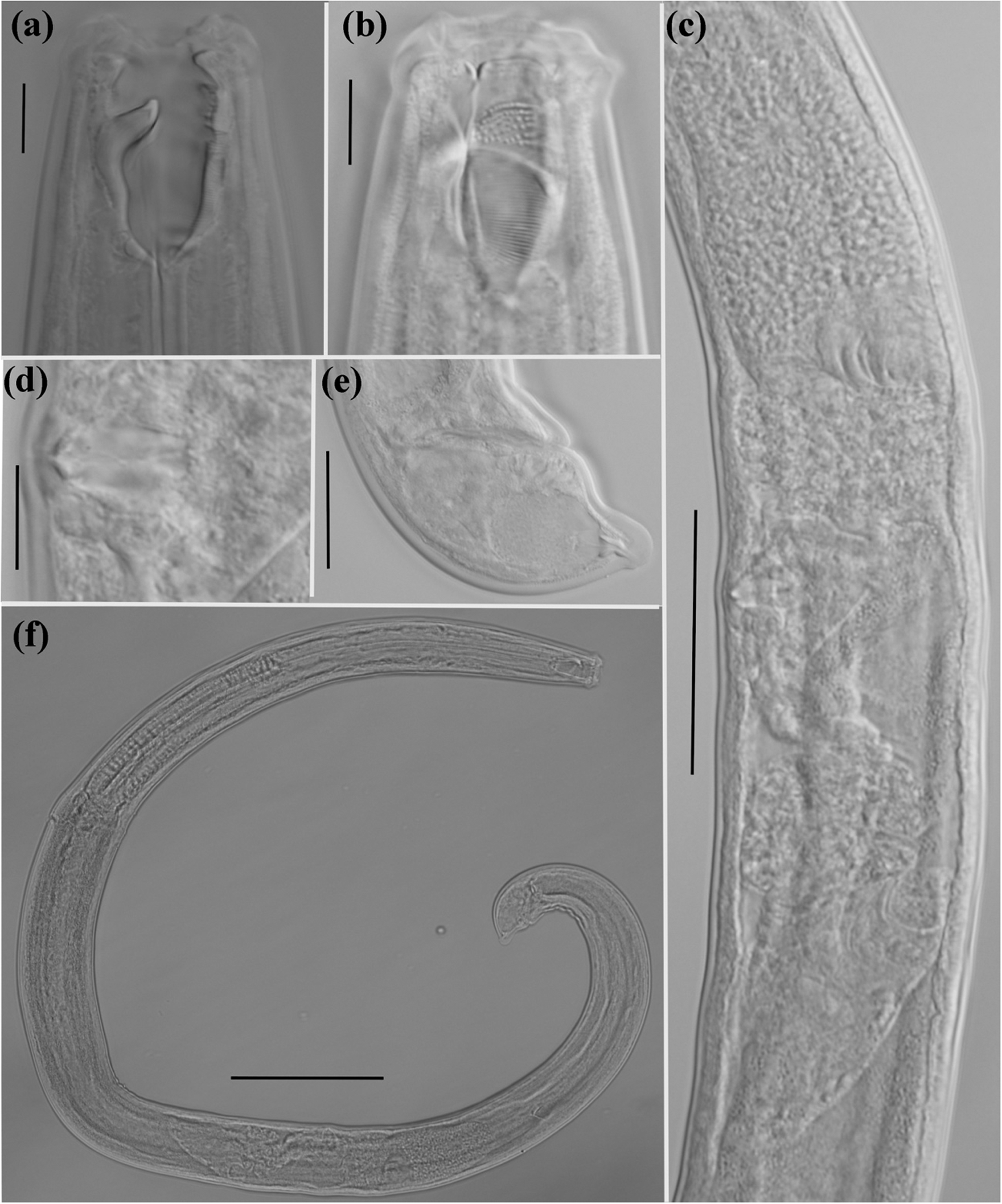
Figure 2. Holotype female of Mylonchulus laocaiensis sp.n. (a): head region; (b): dentical rows; (c): reproductive system; (d): vaginae region; (e): tail region; (f): entire body (scale bars: a, b, d = 10 μm; e = 20 μm; c = 50 μm; f = 100 μm).

Figure 3. Paratype male of Mylonchulus laocaiensis sp. n. (a): head region; (b): dentical rows; (c): spicule gubernaculum and accessory piece; (d): tail region; (e): spermatozoa; entire body (scare bars: a, b, e, f = 10 μm; c = 20 μm; d = 50 μm; g = 100 μm).
Holotype. female, Vietnam, Lao Than mountain, 22°37´14"N; 103°37’24"E, altitude ± 1900 m, Bat Xat Nature Reserve, Lao Cai Province, 12-2021, slide Mylonchulus laocaiensis sp. nov. number 1.
Paratypes. The same data as holotype, 2 male paratypes number 2 and 11. 25 female paratypes on slide numbers 3–10.
Other material. Hoang Lien mountain, 2200 m, Hoang Lien National Park, Lao Cai Province, 9-2022. Twenty-one females on slide numbers 11-20. Holotype female, 15 female paratypes and one paratype males deposited in the nematode collection of the Department of Nematology, Institute of Ecology and Biological Resources of the VAST, Vietnam. Ten female paratypes and one male paratype deposited at the Vietnam National Museum of Nature of the VAST, Vietnam.
Etymology: The name of the species refers to its geographical origin from Lao Cai Province in Vietnam.
Type locality: Lao Cai Province, Vietnam.
Description:
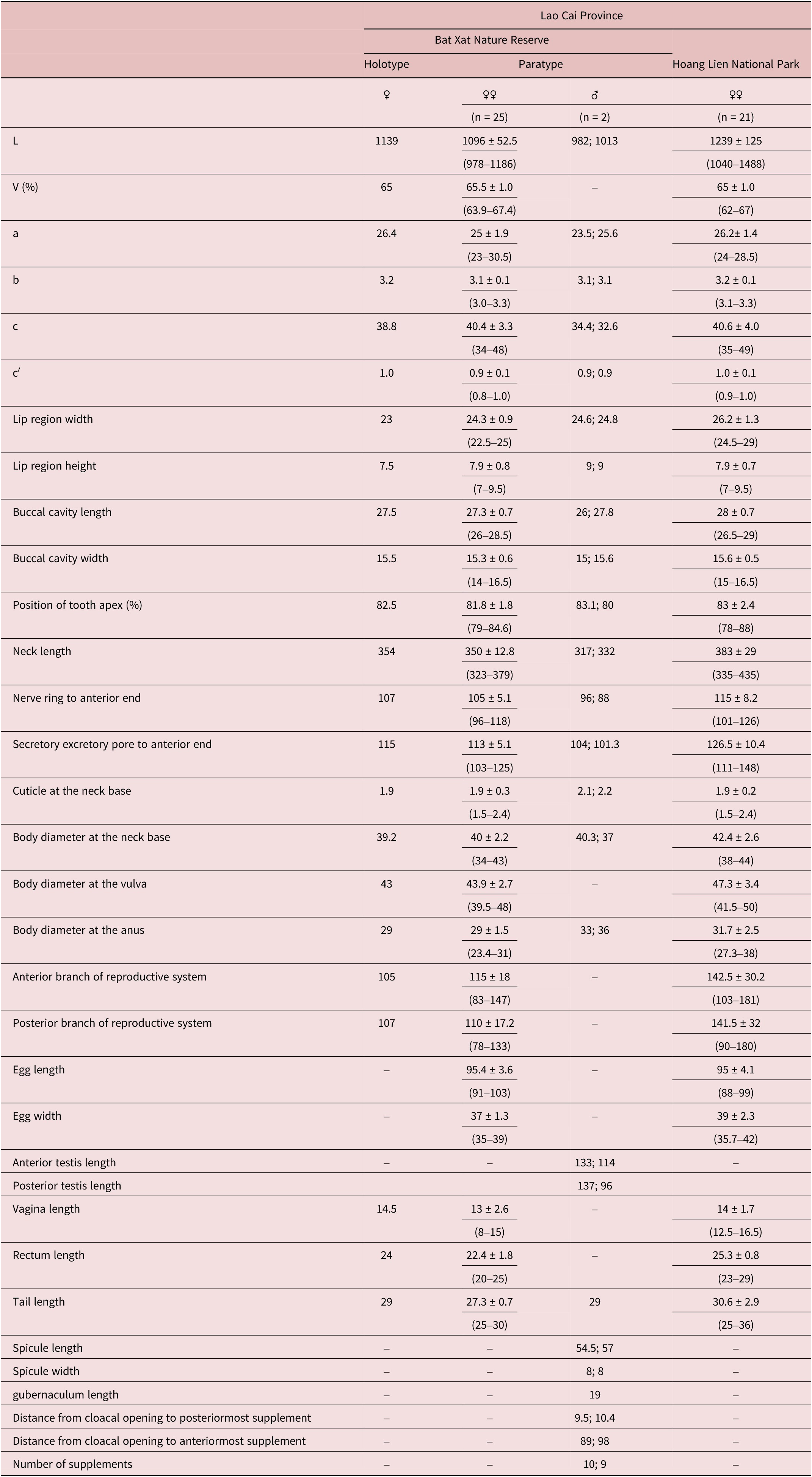
Measurements: see Table 1.
Table 1. Morphometrics of Mylonchulus laocaiensis sp. nov. (all measurements are in μm except indicated and ratios a, b, c, c′)
–, no information.
Adult: Moderately slender to slender (a = 23–30), medium sized nematodes, 1.0–1.5 mm long, habitus curved ventrally and C-shaped after fixation in TAF solution. Body cylindrical, slightly tapering towards the anterior end but more sharply towards posterior end. Maximum body width at level of vulva. Cuticle smooth, two layers, 2.0 (1.5–2.3) μm thick at the base of the pharynx. Lip region angular, prominent set off from body contour by depression, 2.8–3.7 times as wide as high. Lips moderately separated, distinct with prominent labial and cephalic sensilla. Anterior sensilla are arrange in two circles: an anterior one with six inner labials and a posterior one with six outer labials and 4 cephalic papillae slightly protruding beyond the body outline. Amphidial fovea small, fusiform shaped, its aperture oval transverse, 2.2–3 μm wide, located 4.0–5.0 μm from anterior end of buccal cavity or at the level of dorsal tooth apex. Vestibulum 6–7 μm long. Buccal cavity goblet shaped, tapering at base, 1.7–1.9 times as long as wide, its walls heavily cuticularized. Dorsal tooth large, 11–12 μm long from basis to apex, claw-like, forward directed, its apex obtusely, located at anterior fifth (80%–88%) of buccal cavity length from stoma base. A small tooth clearly present in the subventral wall. Five transverse rows of raps-like denticles on subventral walls. Two foramina present at base of buccal cavity lying close to each other. Posterior fourth of buccal cavity embedded in pharyngeal tissue.
Pharynx cylindrical, nerve ring encircling pharynx, located at 30 (28–31)% of neck length. Secretory-excretory pore (SE-pore) indistinct, situated at 33 (31–34)% of neck length. Pharyngo-intestinal junction non-tuberculate, 19–22 × 10–11.5 μm. Rectum straight, shorter than anal body width, 0.8 (0.7–1) times the anal body diameter long. Tail short 0.9–1 time the anal body diameter long; plump, ventrally bent; tail tip rounded. Three caudal glands well developed, lying in tandem and opening sub-terminally on the tail terminus with cuticularized valvular apparatus.
Female: Genital system didelphic-amphidelphic, with both branches equally developed, each branch occupying 11.5 (9.5–13.5)% of total body length. Ovaries reflexed and respectively lengths 55–70 μm with oocytes first arranged in several rows and then in single row on alternate sides of the intestine; sphincter between oviduct and ovary not present. Oviduct 40–70 μm long. Uterus simple, 51–60 μm or 1.3–1.5 body diameter; uterine egg 91–103 × 33–39 μm. Vagina length 13 (8–15) μm occupying 33 (28–40)% corresponding body diameter. Pars proximalis vaginae short; pars refringens vaginae small 2.0 × 1.5 μm, sclerotized in teardrop-shaped pieces; pars distalis vaginae 6–12 × 1.5–2 μm. Vulva transverse, slit-like, at 64%–67% of body length.
Male: Male similar to female in general morphology and body shape and size (see Table 1). Genital system diorchic, with opposed testes. Anterior testis 113–133 μm or 11–13.6% of body length and posterior testis 96–137 μm or 9.5%–14% of body length. Spermatozoa spherical shaped, 2.5–3.5 μm in diameter. Ventromedian supplements 9–10 in number, conical shape, regularly spaced, 80–88 μm apart or occupied 8%–8.5% of body length. The anteriormost supplement situated at 89–98 μm from cloaca aperture. Spicules moderately slender 6.8–7.2 times as long as wide, 54–57 μm long, 8 μm at the maximum width and 1.6–1.8 μm at the minimum width; ventrally curved, with bifurcate terminus, 1.7 times longer than body diameter at cloacal aperture. The head of spicule round, 11 μm long and 5 μm wide. The median piece is 39.5–41 μm long and approximately 4 μm wide. The dorsal hump shallow, curvature 145°. Lateral guiding pieces absent. Gubernaculum well developed, spatuliform in the distal end, 19–20 μm long.
Diagnosis: Mylonchulus laocaiensis sp. n. is characterized by moderately slender to slender body size (1.0–1.5 mm); medium of buccal cavity size (26–29 × 14–16.5 μm); more posterior position of amphidial aperture (4–5 μm) from anterior margin of buccal cavity; 5 denticles rows and small subventral tooth present; pars refringens vaginae small (2.0 × 1.5 μm), cuticularized in teardrop-shaped pieces; tail short (c´= 0.8–1.0); presence of males with short spicules (54–57 μm), rounded head; accessory piece absent; 9–10 lateral supplements regularly arranged.
In general appearance Mylonchulus laocaiensis sp. n. is similar to M. oceanicus Andrássy, Reference Andrássy1986, M. doliolarus Andrássy, Reference Andrássy1992, M. ananasi Yeates, Reference Yeates1992, M. aequatorialis Orselli and Vinciguerra, Reference Orselli and Viciguerra2007, and M. ubis Clark, Reference Clark1961 based on a group of characters: female didelphic, tail shape not broadly rounded, caudal glands well developed with spinneret present on subdorsal tail terminus (Clark, Reference Clark1961; Andrássy, Reference Andrássy1986; Andrássy, Reference Andrássy1992; Yeates, Reference Yeates1992; Orselli and Vinciguerra, Reference Orselli and Viciguerra2007) but differs from these species by the presence of a small subventral tooth and the presence of males.
Mylonchulus laocaiensis sp. n. is distinguished from M. oceanicus by having a more posterior amphidial aperture (at the level of dorsal tooth apex vs at the beginning of anterior buccal cavity); number of denticule rows (5 vs 6–7) and narrower buccal cavity (14–16.5 vs 16–19 μm). Mylonchulus laocaiensis sp. n. differs M. doliolarius Andrássy, Reference Andrássy1992 in body size (1–1.5 μm vs 0.6–0.7 μm); shape of buccal cavity (goblet shaped vs more barrel shaped); anterior dorsal tooth apex (79–88% vs 76–77%); lower c′ value (0.8–1 vs 1) and buccal cavity size (26–29 × 14–16.5 μm vs 16–17 × 14–10 μm). Mylonchulus laocaiensis sp. n. differs from M. ubis by having a longer tail (c = 22–26 vs c = 34–49 and c′ = 1.6 vs c′ = 0.8–1); larger buccal cavity (26–29 × 14–16.5 μm vs 16 × 14–9 μm) and position of anterior amphidial aperture (at level of dorsal tooth apex vs posterior to dorsal tooth apex). Mylonchulus laocaiensis sp. n. can be distinguished from M. aequatorialis by having a higher c value (34–49 vs 24–32) but lower c′ value (0.8–1 vs 1.2–1.4); buccal cavity size (26–29 × 14–16.5 μm vs 20–21 × 8–10 μm) and number of raps-like denticles rows (5 vs 6). From M. ananasi it differs by having a more posterior position of vulva (V = 62–68% vs V = 56–58%); higher c value (34–49 vs 24–27) but lower c′ ratio (0.8–1 vs 1.4–1.7); more anterior position of dorsal tooth apex (79–88% vs 75%) and larger size of buccal cavity (26–29 × 14–16.5 vs 22–24 × 13–14) μm.
Mylonchulus laocaiensis sp. n. is also similar to M. wiliamsi Loof, Reference Loof2006, M. contractus Jairajpuri, Reference Jairajpuri1970, M. brachyuris (Büschli, 1873) Cobb, Reference Cobb1917, M. capsicumi Ishaque et al., Reference Ishaque, Iqbal, Kazi, Dawar and Raza2021 and M. shamimi Handoo et al., Reference Handoo, Kantor and Khan2021 based on group of characters: female didelphic, tail conoid shape, caudal glands well developed with spinneret present on subdorsal tail terminus and having a small subventral tooth (Loof, Reference Loof2006; Jairajpuri, Reference Jairajpuri1970; Cobb, Reference Cobb1917; Ishaque et al., Reference Ishaque, Iqbal, Kazi, Dawar and Raza2021; Handoo et al., Reference Handoo, Kantor and Khan2021).
It differs from M. wiliamsi and M. contractus by having a larger body size (1–1.5 μm vs 09–1 μm and 0.6–0.7 μm); vulva position more posterior (V = 62–68% vs V = 55%–58% and 57%–63%) and shorter tail (c′ = 0.8 vs 1.6–1.9 and 1–1.6). Mylonchulus laocaiensis sp. n. can be distinguished from M. brachyuris, M. capsicumi and M. shamimi by having larger body size (1–1.5 μm vs 0.6–1 μm, 0.9–1 μm); in number of transverse rows of denticles (5 vs 6–7); lower c′ value (0.8–1 vs 1–1.7) and larger buccal cavity (26–29 × 14–6.5 vs 18–25 × 9–15 or 20–22 ×10–12 or 26 × 13).
Mylonchulus laocaiensis sp. n. is also similar to M. hawaiiensis (Cassidy, 1931) Goodey, Reference Goodey1951 and M. lacustris (Cobb, 1915) Andrássy, Reference Andrássy1958 based on group of characters: female didelphic, body length, vulva position, tail conoid shape; having a subventral tooth and presence of males (Goodey, Reference Goodey1951; Andrássy, Reference Andrássy1958). Mylonchulus laocaiensis sp. n. differs M. lactustris by shorter tail (c′ = 0.8 vs 1.7–2.8); spinneret position (subdorsal tail terminus vs central tail terminus) and spicule length (54–57 μm vs 44 μm). Mylonchulus laocaiensis sp. n. can be distinguished from M. haiwaiiensis by number of transverse rows of denticles (5 vs 6); more posterior position of dorsal tooth apex (79–88% vs 69–79%); lower c′ value (0.8–1 vs 1.3–2); spinneret position (subdorsal tail terminus vs central tail terminus) and spicule length (54–57 μm vs 40–44 μm).
Sequence analysis
Molecular sequences of two individuals of Mylonchulus laocaiensis sp. n. were analysed in this study. After sequencing and editing, two sequences were obtained: two 885–932 bp nearly full length of SSU rRNA (18S), were deposited at the GenBank accession number MW544445, MW544446 (sequences were 100% identical) and two 804–824 bp length D2D3 expansion of rRNA (28S), were deposited at the GenBank accession number MW544448, and MW544450 (sequences were 99.8% similar) (Tables 2 and 3).
Table 2. Genetic distances between Mylonchulus laocaiensis sp. n. and other Mylonchulus species using 18S rDNA sequence data (p-distances given in percents)
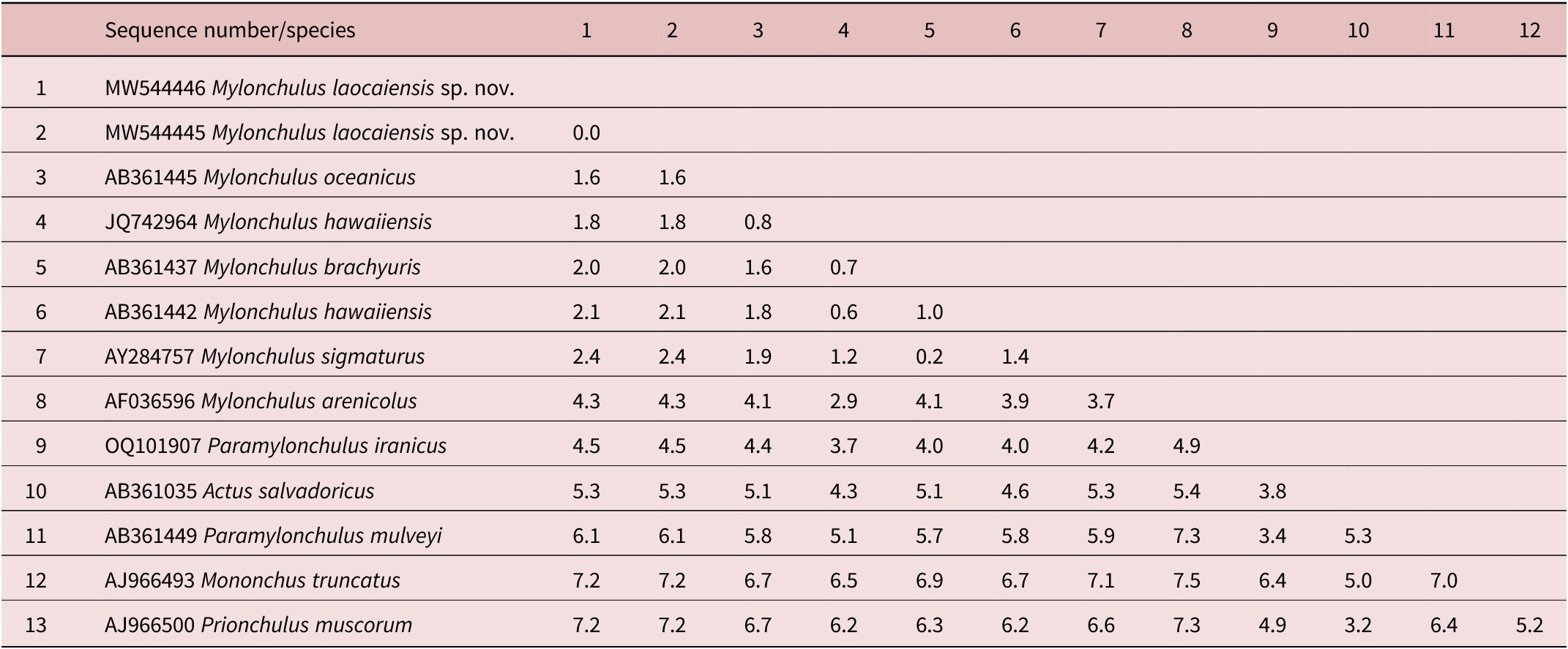
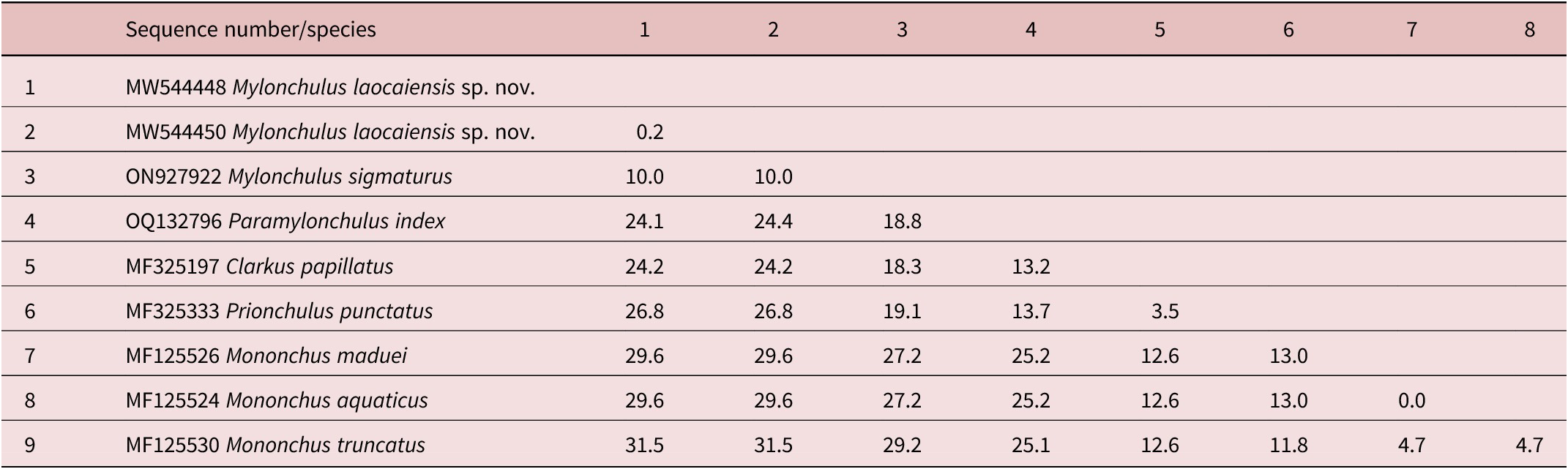
Table 3. Genetic distances between Mylonchulus laocaiensis sp. n. and other Mononchidae species using 28S rDNA sequence data (p-distances given in percents)
The interspecific nucleotide variation within the acquired 18S rDNA sequences was: M. laocaiensis sp. n. vs M. oceanicus (accession number AB361445) =1.6%; vs M. hawaiiensis (accession number JQ742964, AB361441-AB3614453) =1.8-2.1%; vs M. brachyuris (accession number AB361437) = 2.0%; vs M. sigmaturus (AY284756) = 2.4% and vs M. arenicola (accession number AF036596) = 4.1% (Table 2). Furthermore, Mylonchulus laocaiensis sp. n. differs from Paramylonchulus mulveyi (accession number AB361448) = 6.1%; vs P. iranicus (accession number OQ101907) = 4.5% (Table 2).
Based on the 28S rDNA sequences, the dissimilarity of nucleotides between Mylonchulus laocaiensis sp. n. vs M. sigmaturus (accession number ON927922) = 10% and vs Paramylonchulus index (accession number OQ132796) = 24.4% (Table 3).
rDNA phylogenetic relationships among Mononchina
The results derived from the analyses of these 18S and D2–D3 regions of 28S sequences are presented in the molecular trees of Figures 4 and 5, respectively. In the phylogenetic tree of 18S, the new species Mylonchulus laocaiensis sp. n. clustered with the other species of the genus presented, M. oceanicus (AB361443-AB361445), M. hawaiiensis (AB361440-AB361442, JQ742964), M. rotundicaudatus (AY284751), M. sigmaturus (AB361447, AY284755-AY284757, ON927922), M. brachyuris (AB361436, AB361437, AY284754), M. arenicolus (AF036596) and positioned as a clade of Mylonchulus genus. The new species Mylonchulus laocaiensis sp. nov. is sister to M. oceanicus but they are clearly separated, with high genetic distance (Figure 4). Mylonchulus species were grouped together with genera Paramylonchulus (Paramylonchulus mulveyi (AB361448, AB361449), P. iranicus (OQ101907)) and presented as subfamily Mylonchulinae. The subfamily Mylonchulinae is sistered with subfamily Sporonchulinae (Sporonchulus (S. ibitiensis (accession number OQ377123)) and Actus (A. salvadoricus accession number AB361035)). This phylogenetic tree to those reported by Olia et al. (Reference Olia, Ahmad, Araki, Minaka, Oba and Okada2008), Aliramaji et al. (Reference Aliramaji, Taheri and Shokoohi2023), Singh et al. (Reference Singh, Singh, Singh, Singh and Meitei2023), Vu et al. (Reference Vu, Nguyen and Peña-Santiago2024). Conversely, 28S tree presents Mylonchulinae sequences (Mylonchulus laocaiensis sp. n. (accession number MW544448-544450), M. sigmaturus (accession number ON927922), Mylonchulus sp_MZM (accession number OQ223303) and Mylonchulus (Paramylonchulus) index (accession number OQ132796)) forming a 100% supported clade Mylonchulidae, which is the sister group of the remaining taxa included in another (Figure 5). Both species of the genus Mylonchulus (Mylonchulus laocaiensis sp. n. and M. sigmaturus) differ from Mylonchulus (Paramylonchulus) index by 18.8%–24.4% base pairs (Table 3) which supports well the validity of the genus Paramylonchulus (following Aliramaji et al., Reference Aliramaji, Taheri and Shokoohi2023). The second major clade therefore consists of four subclades, namely (((Mononchinae + ((Sporonchulinae + (Anatonchidae + Prionchulinae))), but the resolution is not totally satisfactory as the clade ((Sporonchulinae + (Anatonchidae + Prionchulinae)) presents low support. The same results were reported by Singh et al. (Reference Singh, Singh, Singh, Singh and Meitei2023) and Vu et al. (Reference Vu, Nguyen and Peña-Santiago2024). The analysis of D2-D3 regions of 28S sequences are always more believable than 18S because they have mutations on these regions. Neither of the trees confirms the systematic division of Mononchina suborder into two superfamilies: Mononchoidea and Anatonchoidea, a system that have been accepted until now (Jairajpuri, Reference Jairajpuri1969; Andrássy, Reference Andrássy1976, Reference Andrássy2009). Interestingly, is that not only species of family Mylonchulidae (genera Mylonchulus and Paramylonchulus), but also some other genera such as Mulveyellus and Jensenonchus (family Anatonchidae) did not place in the family based on the 28S tree (Singh et al., Reference Singh, Singh, Singh, Singh and Meitei2023; Vu et al., Reference Vu, Nguyen and Peña-Santiago2024). The authors believe that the shape of buccal cavity is an important characteristic for the division of the suborder Mononchina rather than the non-tuberculate or tuberculate pharyngo-intestinal junction. Thus, further molecular research should be conducted to elucidate this matter.

Figure 4. Bayesian Inference tree from the newly sequenced Mylonchulus laocaiensis sp. n. based on sequences of the 18S rDNA region. Bayesian posterior probabilities (%) are given for each clade. Scale bar shows the number of substitutions per site.

Figure 5. Bayesian Inference tree from the newly sequenced Mylonchulus laocaiensis sp. n. based on sequences of the 28S rDNA region. Bayesian posterior probabilities (%) are given for each clade. Scale bar shows the number of substitutions per site.
Acknowledgement
This study is a part of research project “Studies on selected nematode taxa of the orders Mononchida and Dorylaimida from Vietnam and Bulgaria”, grant number QTBG01.04/23-24 funded by the Vietnam Academy of Science and Technology (VAST).
Author contribution
Vu T.T.T. and Peneva V. analysed morphometric data, morphological characteristic, gave a line-drawing and photos, prepared and submitted manuscript. Le T.M.L. analysed DNA data. All authors are in agreement about the content of the manuscript.
Data availability statement
Holotype, 15 female paratypes and one male paratyle have been deposited at the Department of Nematology, Institute of Ecology and Biological Resources, Vietnam. Ten female paratypes and one male paratype deposited at the VNMN of the Vietnam Academy of Science and Technology (VAST), Vietnam. The DNA sequence have been deposited in the GenBank database.
Competing interest
Authors declare that they have no competing interests.
Ethics approval consent to participate
Not applicable.
Consent for publication
Not applicable.










