Introduction
Conservation of biological diversity is a global concern. Ecosystems have experienced increasing anthropogenic disturbance in structure and function, resulting in alterations to species composition and abundance (Turner, Reference Turner1996; Laurance, Reference Laurance1999; Scott et al., Reference Scott2006). The remnant natural environments in the Brazilian Atlantic Forest have been dramatically reduced, fragmented or isolated due to a continuous process of urbanization and development that has caused habitat changes. The Atlantic Forest is located in the most densely populated region in Brazil. This biome, considered a hotspot of biodiversity, is characterized by its habitat heterogeneity (Caramaschi et al., Reference Caramaschi, Bergallo, Rocha, Alves and Van Sluys2000), its isolation from other ombrophilous forests and its high degree of fragmentation.
Within the Atlantic Forest biome, the state of Rio de Janeiro has a wide mammalian fauna (Rocha et al., Reference Rocha2003), but in several localities the medium and large animals have disappeared or have very small populations. This decline has led to changes in ecological interactions, an abundance of generalist small mammal species, and an increase in the presence of many species around human dwellings, such as the black-eared opossum Didelphis aurita (Didelphimorphia, Didelphidae) (Fonseca and Robinson, Reference Fonseca and Robinson1990; Moura et al., Reference Moura, Vieira and Cerqueira2009).
Human infection by the parasitic helminths of wild animals has been attributed to the increasing interface of urban or rural populations with sylvatic areas, where mammalian species may act as reservoirs of zoonosis (Gentile et al., Reference Gentile2006; Simões et al., Reference Simões2014). Mammalian helminth infections may also occur in domestic, synanthropic and breeding animals (Prestwood et al., Reference Prestwood, Nettles and Farrell1977; Simões et al., Reference Simões2011; Brianti et al., Reference Brianti2012).
The community structure of parasites is usually studied at the infracommunity (individual hosts) and component-community (set of local infracommunities) levels (Poulin and Dick, Reference Poulin and Dick2007). A recent approach to the study of community structure uses metacommunity analysis (Winegardner et al., Reference Winegardner2012). A metacommunity can be defined as a set of local communities potentially linked by the dispersal of multiple species on a larger spatial scale (Gilpin and Hanski, Reference Gilpin and Hanski1991; Leibold et al., Reference Leibold2004). The analysis of the Elements of Metacommunity Structure (EMS hereafter) evaluates three elements. Coherence is a measure that tests whether species respond to the same environmental gradient, that is, the degree to which a pattern can be grouped into a single dimension, and is quantified by the number of interruptions in the occurrence of a species (embedded absences) in an incidence matrix. The second element, species turnover, represents the number of species replacements in this dimension. The boundary clumping element represents how the limit of occurrence of each species is distributed between the sites along the environmental gradient (Leibold and Mikkelson, Reference Leibold and Mikkelson2002). Six idealized basic structures can be identified: checkerboard, nested, Clementsian, Gleasonian, evenly spaced and random distributions (Leibold and Mikkelson, Reference Leibold and Mikkelson2002). This is a promising approach for studies of parasite communities (Richgels et al., Reference Richgels, Hoverman and Johnson2013) or zoonotic diseases (Suzán et al., Reference Suzán2015) due to the fragmented nature of the populations of these organisms, where each individual host comprises an infracommunity.
Previous studies on the helminths of D. aurita have primarily been taxonomic descriptions and records of occurrence (Thatcher, Reference Thatcher, Cáceres and Monteiro Filho2006; Chagas-Moutinho et al., Reference Chagas-Moutinho2014). There have been a few studies on helminth community structure in marsupial species (Antunes, Reference Antunes2005; Jiménez et al., Reference Jiménez, Catzeflis and Gardner2011; Byles et al., Reference Byles2013). This study describes the species composition and analyses the structure of the helminth metacommunities of D. aurita at both infracomunity and component community levels. We compare the abundance, intensity, prevalence and richness of the helminth species in peri-urban, sylvatic and rural habitats in the state of Rio de Janeiro and investigate the parasitological parameters for the most abundant species. We hypothesized that, for this host, the helminth metacommunity across infracommunities and component communities on a regional scale would have a coherent pattern of species distribution. In this way, species would be non-randomly distributed along the environmental gradient formed by hosts and the external habitats of each area due to the heterogeneity in the host exposure to helminth species among habitats.
Material and methods
Study area
This study was carried out in three habitat types within the Atlantic Forest in the state of Rio de Janeiro: peri-urban, sylvatic and rural. The peri-urban environment comprised localities of the Fiocruz Atlantic Forest Campus (CFMA) (22°56′18.00″S, 43°24′10.13″W) and the Pedra Branca State Park (PBSP) subunit Pau-da-Fome (22°56′55.32″S, 43°26′36.66″W) in the Municipality of Rio de Janeiro. The CFMA partially overlaps with the PBSP, and both are adjacent to an expanding urban region in the Jacarepaguá Basin that encompasses preserved areas. The PBSP is considered the largest forest reserve located within an urban environment in the Americas, covering 12,492 hectares. The predominant vegetation is ombrophilous dense forest. This area was previously occupied by sugar cane mills and coffee farms. During the last decade, the area began to suffer greater anthropogenic pressure as a result of an increasing population, the expansion of low-sanitation communities, the implementation of major infrastructure projects and the expansion of road networks. All samplings were conducted near dwellings and in disturbed forest areas.
The sylvatic environment comprised three localities in the Serra dos Órgãos National Park (PARNASO), Bonfim (22°27′50.53″S, 43°05′17.78″W), Uricanal (22°29′04.99″S, 43°07′02.50″W) and Barragem-do-Caxambú (22°30′09.46″S, 43°07′12.33″W) in the municipality of Petrópolis. PARNASO encompasses 20,024 hectares and is located in the mountain region of the state of Rio de Janeiro, c. 80 km from the city of Rio de Janeiro. It includes four municipalities (Teresópolis, Petrópolis, Magé and Guapimirim). The present study was carried out in the municipality of Petrópolis. The park is one of the most important Atlantic Forest remnants in the state of Rio de Janeiro and is internationally recognized as a Biosphere Reserve. Samplings were conducted in areas of preserved dense ombrophilous montane forest in Uricanal and Barragem-do-Caxambú. Bonfim is also within a preserved ombrophilous forest, but it is located closer to human lodgings.
The rural environment comprised the localities of Pamparrão (22°01′47.03″S, 42°39′13.46″W) and Porteira Verde (22°02′20.57″S, 42°39′23.53″W) in the municipality of Sumidouro, in the mountain region of the state of Rio de Janeiro, c. 160 km from the city of Rio de Janeiro. Since the 19th century, the economy of this region has been based on agriculture and livestock. Currently, agriculture is the main income source in the region, especially vegetable cultivation and milk production. The area still has some Atlantic Forest remnants of dense ombrophilous forest, mostly in the high mountain ranges. Anthropogenic activities have transformed the landscape into a matrix of agricultural plots and pastures, with small fragments of forest remnants. Samplings were carried out in plantations, pastures and along shrub vegetation.
Host captures
The marsupials were collected in Tomahawk® live-traps (16 × 5 × 5 inches). The collections were made between 2012 and 2015 in wet and dry seasons in forested areas. Each sampling was conducted for five consecutive days in the peri-urban and rural areas and for ten days in the sylvatic area. The traps were placed on the ground in transects of 20 points. All traps were baited with a mixture of peanut butter, banana, oatmeal and bacon. The capture effort included 4560 trap-nights in the peri-urban environment, 2160 trap-nights in the sylvatic area and 1330 trap-nights in the rural area, for a total of 8050 trap-nights.
The animals were captured under the authorization of the Brazilian Government's Chico Mendes Institute for Biodiversity and Conservation (ICMBio, license numbers 13373 and 45839-1) and the Environmental Institute of Rio de Janeiro State (INEA, license number 020/2011). All procedures followed the guidelines for capture, handling and care of animals from the Ethical Committee on Animal Use of the Oswaldo Cruz Foundation (CEUA license numbers L-049/08, LW-81/12 and LW-39/14). Biosafety techniques and personal safety equipment were used during all procedures involving animal handling and biological sampling.
Helminth recovery and identification
The viscera, thoracic and abdominal cavities, pelvis and musculature of the marsupials were examined for helminths. The helminths were separated in Petri dishes with saline (0.85% NaCl) and dissected under a stereoscopic microscope. The helminths were fixed in AFA solution (93 parts 70% ethanol, 5 parts 0.4% formol and 2 parts 100% acetic acid) and heated to 65°C. Some specimens were stored in 70% ethanol for further molecular analysis. The trematodes were fixed in the same solution under compression, and the cestodes and acanthocephals were kept in distilled water for relaxation of the musculature (Amato et al., Reference Amato, Walter and Amato1991). Some of the nematode specimens were diaphanized with lactophenol or glycerinated alcohol. Some of the trematodes, cestodes and acanthocephals were stained with Langeron's carmine or Delafield's haematoxylin, differentiated with 0.5% hydrochloric acid, dehydrated in a crescent alcoholic series, diaphanized in methyl salicylate and fixed in Canada balsam for a permanent preparation (Amato et al., Reference Amato, Walter and Amato1991). Subsequently, the specimens were analysed with a light microscope (an Axio Scope.A1-Zeiss coupled to an Axio Cam MRc digital camera) for photomicrography. The specimens were counted and identified using morphological aspects according to Travassos (Reference Travassos1937), Yamaguti (Reference Yamaguti1961), Khalil et al. (Reference Khalil, Jones and Bray1994), Vicente et al. (Reference Vicente1997), Gibson et al. (Reference Gibson, Jones and Bray2002), Jones et al. (Reference Jones, Bray and Gilbson2005), Anderson et al. (Reference Anderson, Chaubaud and Willmott2009) and other articles of species description.
Voucher specimens of the opossums were deposited at the National Museum of Rio de Janeiro, and specimens of the helminths were deposited at the Helminthological Collection of the Oswaldo Cruz Institute (38321, 38322, 38323, 38571, 38572, 38573, 38574, 38575, 38576, 38577, 38578, 38579).
Data analysis
Parasitological parameters were calculated for each helminth species according to Bush et al. (Reference Bush1997). The mean abundance was considered as the total number of helminths of a certain species divided by the number of hosts analysed. The mean intensity was calculated as the total number of helminths of a species divided by the number of animals infected by the species. Prevalence was calculated as the proportion of infected animals in relation to the total number of animals analysed. The spatial distribution of the helminths in the host populations was calculated using the variance-to-mean ratio of the abundance of each helminth species, but only for species with prevalence > 10%. The mean species richness was calculated for each municipality as the mean number of species infracommunities for each area.
The abundance, intensity and prevalence rates were compared for the most abundant species according to the habitat type where the host was captured (peri-urban in the municipality of Rio de Janeiro, rural in the municipality of Sumidouro and sylvatic in the municipality of Petrópolis), the host gender (male or female) and the season (dry or rainy). Prevalence, intensity and abundance were compared using generalized linear models (GLM), when the data were available. The best models were chosen using the corrected Akaike information criterion (AICc), which considered a model plausible if it had ΔAICc ≤ 2. Only the models with significant effects were considered.
Synergism or segregation between helminth pairs of species was investigated using the Spearman correlation only for the most abundant species that had similarities in their life cycles and sites of infection in the host, indicating similar niches. An importance index was calculated according to Thul et al. (Reference Thul1985) to characterize each species in the community. Each helminth species was classified in the community as dominant (I ≥ 1.0), co-dominant (0.01 ≤ I ≤ 1.0) or subordinate (I < 0.01).
Similarities in helminth species composition and richness among localities (CFMA, Pau-da-Fome, Uricanal, Bonfim, Barragem-do-Caxambú, Pamparrão and Porteira Verde) were evaluated using the non-metric Multidimensional Scaling (nMDS) analysis. In this analysis, the species presence or absence and the Jaccard distance measure were used.
The structure of the helminth metacommunity was evaluated on a regional scale in relation to infracommunity, considering each infected host as a site, and to component community, considering each locality as a subset of hosts. These analyses were performed including all infected animals of the entire study using the EMS analysis. The structure of the metacommunity was also analysed separately on a local scale for each municipality (Rio de Janeiro – peri-urban; Petrópolis – sylvatic; Sumidouro – rural), only in relation to infracommunity in order to compare with the regional metacommunity structure. The three elements of metacommunity structure were evaluated according to Leibold and Mikkelson (Reference Leibold and Mikkelson2002) and Presley et al. (Reference Presley, Higgins and Willig2010). In the EMS analysis, when coherence is significantly positive (number of embedded absences larger than that of the randomly generated matrix), the other parameters are also analysed. When the coherence is not significant, the distribution of the species in the environmental gradient is random. When coherence is significantly negative (number of embedded absences less than that of the randomly generated matrix), a checkerboard distribution is observed (Leibold and Mikkelson, Reference Leibold and Mikkelson2002). Nested, Clementsian, Gleasonian and evenly spaced structures are based on the significance of the turnover and boundary clumping, and whether these elements are positive or negative (Leibold and Mikkelson, Reference Leibold and Mikkelson2002).
Chi-square tests and nMDS analysis were performed using the statistics package R (R Core Team, 2017), and the GLM analysis used the vegan package (Oksanen et al., Reference Oksanen2017) in RStudio software version 1.0.136. The metacommunity structure analysis used MATLAB R2017B software (MathWorks Inc., Naticks, MA, USA; EMS script available at http://faculty.tarleton.edu/higgins/metacommunity-structure.html). The level of significance was 5% in all the analyses.
Results
During the study, 73 specimens of D. aurita were captured, 48 in the peri-urban environment, 13 in the sylvatic areas and 12 in the rural areas. The helminth richness was composed of 14 species belonging to three phyla. Nine species of the phylum Nematoda were found: Aspidodera raillieti Travassos, 1913 (Ascaridida, Aspidoderidae), Cruzia tentaculata (Rudolphi, 1819) Travassos, 1922 (Ascaridida, Kathlaniidae), Trichuris minuta Rudolphi, 1819 (Trichocephalida, Trichuridae) and Trichuris didelphis Barero, 1959 (Trichocephalida, Trichuridae) in the large intestine; Travassostrongylus orloffi Travassos, 1935 (Rhabditida, Viannaiidae), Viannaia hamata Travassos, 1914 (Rhabditida, Viannaiidae) and Globocephalus marsupialis Freitas & Lent, 1936 (Rhabditida, Ancylostomatidae) in the small intestine; Heterostrongylus heterostrongylus Travassos, 1925 (Rhabditida, Angiostrongylidae) in the bronchus and pulmonary bronchioles; and Turgida turgida (Rudolphi, 1819) Travassos, 1919 (Spirurida, Physalopteridae) in the stomach. Four species of the phylum Platyhelminthes were identified: three trematode species, Duboisiella proloba Baer, 1938 (Strigeidida, Strigeidae), Brachylaima advena Dujardin, 1843 (Brachylaemiformes, Brachylaimidae) and Rhopalias coronatus (Rudolphi, 1819) Stiles; Hassall, 1898 (Plagiorchiida, Rhopaliidae) in the small intestine, and one species of Cestoda, which was not identified, also located in the small intestine. One species of the phylum Acanthocephala was found in the small intestine, Oligacanthorhynchus microcephalus (Rudolphi, 1819) Schmidt, 1972 (Archiacanthocephala, Oligacanthorhynchidae). The species richness was highest in the peri-urban environment (s = 5.32, total = 13 species), followed by the sylvatic environment (s = 3.92, total = 12 species) and the rural environment (s = 2.08, total = 5 species).
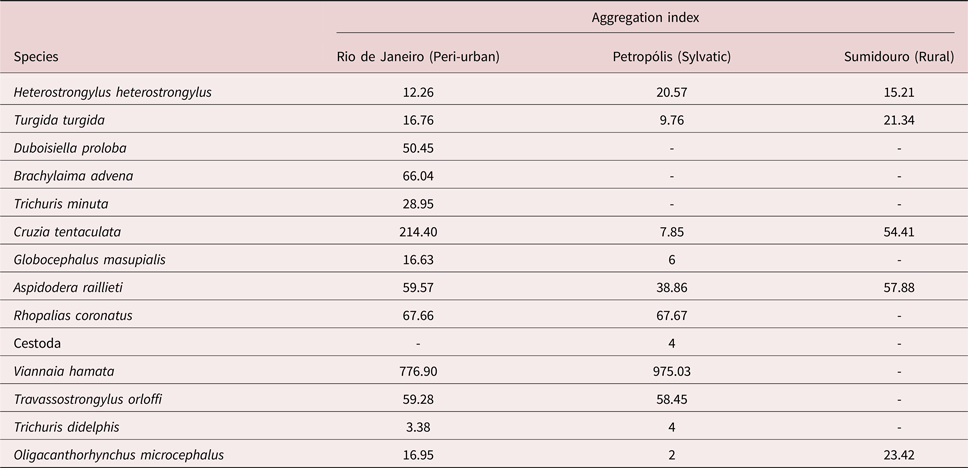
No host was infected with all helminth species; only 4.1% were not infected by any helminth species. A total of 145 adult helminths were recovered from H. heterostrongylus, 615 from T. turgida, 3660 from C. tentaculata, 1268 from A. raillieti, 5679 from V. hamata, 1065 from T. orloffi and 112 from O. microcephalus. Viannaia hamata and C. tentaculata were the most abundant species of the study. The most prevalent species were C. tentaculata and T. turgida (table 1). The seven most abundant species were highly aggregated relative to each studied habitat type (table 2).
Table 1. Intensity, abundance (± SD) and prevalence (95% confidence interval) of the helminth species of Didelphis aurita in the state of Rio de Janeiro, Brazil, in relation to habitat, host gender and season.

Table 2. Aggregation indices of each helminth species of Didelphis aurita for each habitat in the state of Rio de Janeiro, Brazil.
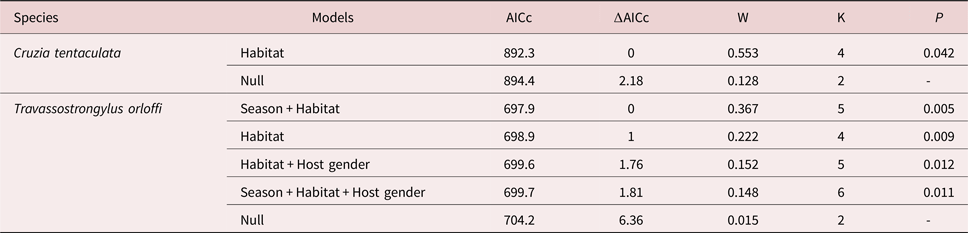
Heterostrongylus heterostrongylus had significant differences in prevalence in relation to habitat with higher values in the sylvatic environment (tables 1 and 3). Cruzia tentaculata was the second most abundant species, with its abundance being significantly higher in the peri-urban habitat (tables 1 and 4). Statistically significant differences were observed in abundance and prevalence of T. orloffi in relation to host, habitat and season, with the abundance and prevalence being higher for males, in the peri-urban habitat and in the dry season (tables 1, 3 and 4). Prevalence of V. hamata was significantly higher in male hosts and in the peri-urban habitat (tables 1 and 3). In O. microcephalus, prevalence was significantly higher in females and in the peri-urban habitat (tables 1 and 3). For T. turgida and A. raillieti, no statistically significant differences were found in relation to the analysed parameters.
Table 3. Generalized linear models for the helminth prevalences in relation to habitat, host gender and season for helminth species of Didelphis aurita in the state of Rio de Janeiro, Brazil. AICc, Akaike information criterion corrected for small sample size; ∆AICc, difference between the AICc of a model and the model of lowest AICc; W, Akaike weight; K, number of parameters; P, significance level of the model.

Table 4. Generalized linear models for the helminth abundances in relation to habitat, host gender and season for helminth species of Didelphis aurita in the state of Rio de Janeiro, Brazil. AICc, Akaike information criterion corrected for small sample size; ∆AICc, difference between the AICc of a model and the model of lowest AICc; W, Akaike weight; K, number of parameters; P, significance level of the model.
The sex ratios of T. turgida, H. heterostrongylus and O. microcephalus did not differ from 1 : 1. Cruzia tentaculata, A. raillieti, V. hamata and T. orloffi had significantly more females than males (χ2 = 61.48, P < 0.0001; χ2 = 14.25, P = 0.0001; χ2 = 549.02, P = 0.0001; χ2 = 32.45, P = 0.0001, respectively).
Helminth abundance correlations were analysed between C. tentaculata and A. raillieti, collected in the large intestine, and between V. hamata and T. orloffi, collected in the small intestine, all presenting direct life cycles. Only V. hamata and T. orloffi showed a co-occurrence in the hosts (rs = 0.50, P ≤ 0.0001, N = 47).
The nMDS analysis indicated that, except for the rural locality Porteira Verde in the municipality of Sumidouro, localities with more anthropogenic impact were grouped in the centre of the plot. Those localities set in the margins of the plot represented environments with lower levels of disturbance, such as Barrragem-do-Caxambú and Uricanal in PARNASO (fig. 1).

Fig. 1. Similarity in species composition among localities for helminths of Didelphis aurita in the state of Rio de Janeiro, Brazil, using non-metric multidimensional scaling (nMDS).
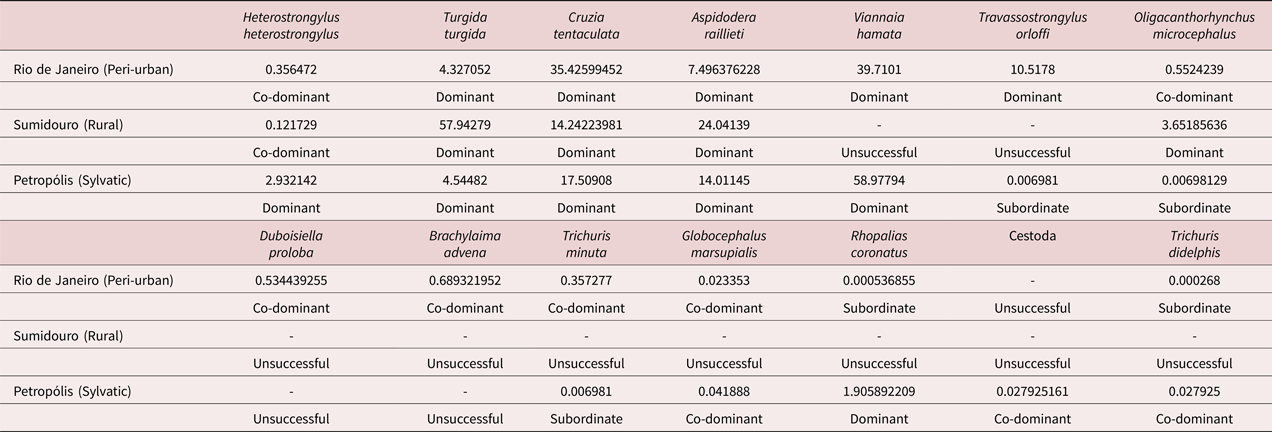
The nematodes T. turgida, C. tentaculata and A. raillieti were dominant in all habitats, while the other helminths were either co-dominant or subordinate (Table 5). Heterostrongylus heterostrongylus was dominant only in the sylvatic habitat; V. hamata was dominant in the sylvatic and peri-urban environments; T. orloffi was dominant only in the peri-urban environment; O. microcephalus was dominant only in the rural environment; and R. coronatus was dominant only in the sylvatic habitat (table 5).
Table 5. Importance indices of each helminth species of Didelphis aurita for each habitat in the state of Rio de Janeiro, Brazil.
The metacommunity analysis indicated that the helminth species showed a coherent structure on the regional scale (table 6). Considering all infracommunities, we observed a quasi-nested structure with stochastic species loss (species turnover and boundary clumping were not significant) (table 6, fig. 2a). Considering all component communities, the observed structure was nested with stochastic species loss (significant negative species turnover and non-significant boundary clumping) (table 6, fig. 2b). On both analyses, species-poor sites had subsets of species-rich sites (fig. 2a, b). Considering each municipality separately, the structure of the metacommunities at the infracommunity level was random in Rio de Janeiro and Sumidouro (urban and rural areas) (non-significant coherence) and revealed a checkerboard structure in Petrópolis (sylvatic area) (significant negative coherence) (table 6).
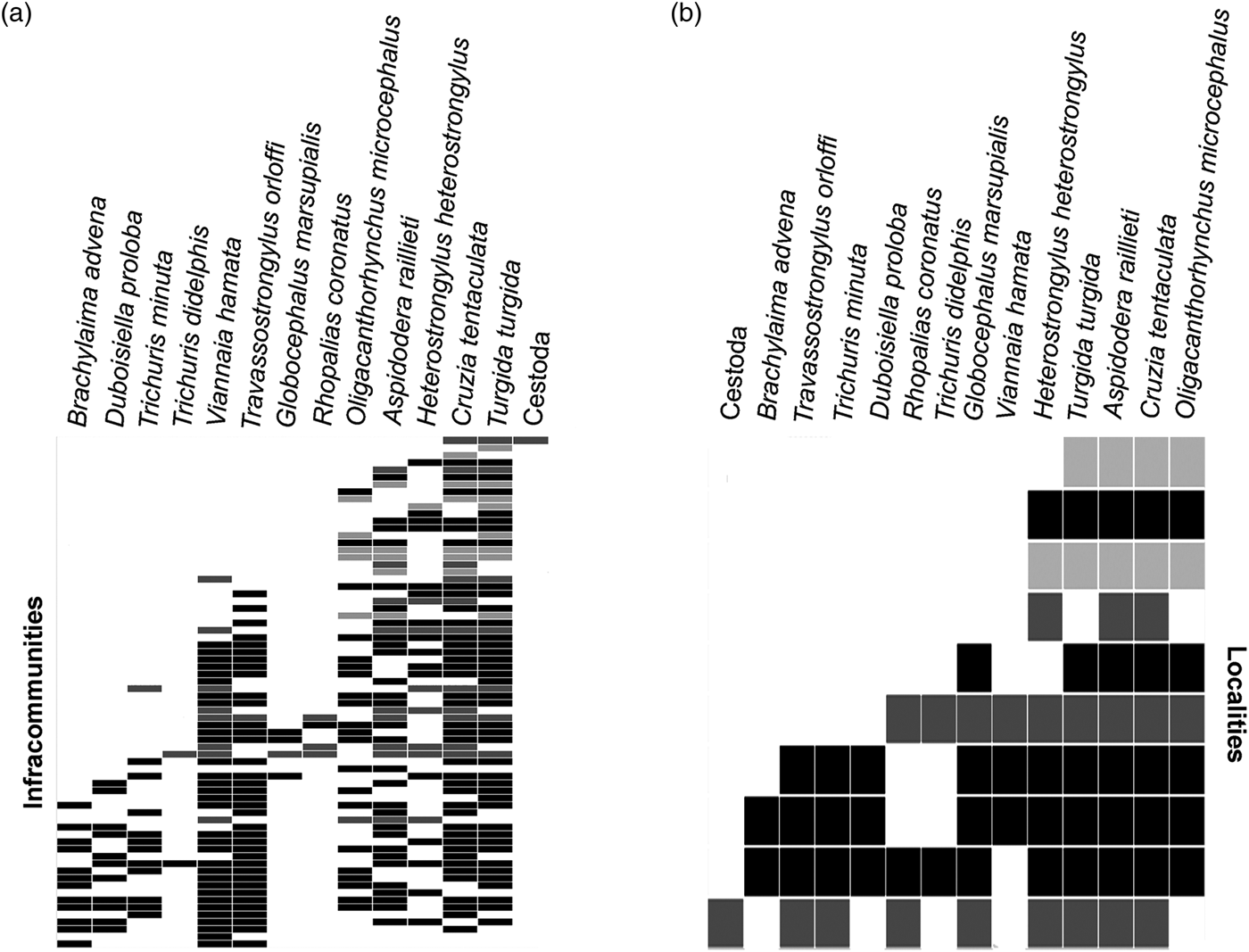
Fig. 2. Ordinated matrices for helminth metacommunities, on the infracommunity and component community levels, of Didelphis aurita in Rio de Janeiro, Petrópolis and Sumidouro municipalities. (a) Infracommunity level; the lines correspond to the host specimens, and columns correspond to the helminth species. (b) Component community level; the lines correspond to the localities, and columns correspond to the helminth species. Rio de Janeiro–RJ (black: CFMA includes component communities in three areas and PBSP in two areas) Petrópolis–RJ (dark grey) and Sumidouro–RJ (light grey). Embedded absences were not filled.
Table 6. Results of the analysis of the elements of metacommunity structure for the helminth metacommunity recovered from Didelphis aurita in Rio de Janeiro, Petrópolis and Sumidouro municipalities, state of Rio de Janeiro, Brazil. Abs, embbeded absences; SD, standard deviation; Rep, replacements; IM, Morisita's index.

Discussion
This is the first study of the helminth community structure of D. aurita. One previous study reported the structure of helminth communities for another species of the genus Didelphis; it was carried out in French Guiana on the component community level (Jiménez et al., Reference Jiménez, Catzeflis and Gardner2011). The present study represents a pioneering investigation of the metacommunity structure of the helminths of a Neotropical marsupial.
The most abundant species in the present study (V. hamata, C. tentaculata, A. raillieti and T. orloffi) have also been found in other studies of helminth fauna of the genus Didelphis. Antunes (Reference Antunes2005) found C. tentaculata to be the most abundant and prevalent species in Didelphis albiventris in Rio Grande do Sul, followed by A. raillieti, T. turgida and V. hamata. Cruzia tentaculata was also the most abundant species in D. aurita in the state of Rio de Janeiro, followed by A. raillieti, V. hamata and T. turgida in a study by Gomes et al. (Reference Gomes2003). Silva and Costa (Reference Silva and Costa1999) found C. tentaculata, A. raillieti, V. hamata, T. orloffi, T. turgida, T. didelphis and R. coronatus in D. albiventris in Belo Horizonte. Jiménez et al. (Reference Jiménez, Catzeflis and Gardner2011) found A. raillieti and C. tentaculata to be the most abundant species of Didelphis marsupialis in French Guiana.
Didelphis aurita is a new host for the nematodes G. marsupialis and T. didelphis. This study provides the first record of the species G. marsupialis, H. heterostrongylus, T. orloffi, T. minuta, T. didelphis and D. proloba in Rio de Janeiro, the first record of A. raillieti, C. tentaculata, H. heterostrongylus, T. orloffi, T. minuta, T. didelphis, T. turgida, V. hamata and O. microcephalus in Petrópolis, and the first record of H. heterostrongylus and O. microcephalus in Sumidouro.
The greater abundance of females observed in C. tentaculata, A. raillieti, V. hamata and T. orloffi suggests an ecological strategy to their reproduction. Increased egg production could contribute to the host infection (Oliveira-Menezes et al., Reference Oliveira-Menezes, Lanfredi-Rangel and Lanfredi2011). In polygamous mating systems, sex ratios favouring females may increase the chances of mating (Poulin, Reference Poulin2007).
All the helminth species analysed in this study showed high aggregation rates. Heterogeneity among hosts in relation to parasite exposure is considered to be one of the main factors associated with parasite aggregation (Poulin, Reference Poulin2013).
The nested and quasi-nested results obtained for the metacommunity structure considering component communities and infracommunities, respectively, indicate that areas with a lower species richness form subsets of areas with high species richness. In species-poor sites, hosts share fewer parasites among themselves, and in species-rich sites, there is a sharing of a greater diversity of parasites. The quasi-nested structure indicates a weaker structuring pattern than the nested one, but with the same characteristics. A predictable pattern of species loss among sites characterizes a nested structure, in this case, when a species is absent from a particular site, it is predicted to be absent from all sites with fewer species (Presley et al., Reference Presley, Higgins and Willig2010). The nested pattern is commonly observed in parasite communities (van der Mescht et al., Reference van der Mescht2016) and indicates that hosts accumulate parasite species gradually and predictably (Poulin, Reference Poulin2007).
The nMDS analysis indicated that closer localities in the plot represent greater similarities in species composition, suggesting that the clustered localities may have common ecological characteristics that favour the presence of the helminth species. The analysis revealed a central grouping of localities with urban–sylvatic interface environments. Moreover, the nMDS also indicated lower diversity of species among environments. Thus, according to both analyses, nMDS and EMS, the component community of the rural area represented a subset of the component communities of the peri-urban and sylvatic areas. This pattern was also reflected in the infracommunities. The occurrence of dominant species with higher abundance and prevalence, such as C. tentaculata, T. turgida and A. raillieti, in the localities with lower richness indicates that these parasites belong to the core of the component communities. Species such as G. marsupialis, R. coronatus, T. didelphis and O. microcephalus occurred in some of the communities and were usually subordinate or co-dominant, forming an intermediate group in the nested pattern. A third group of species, including D. proloba, B. advena and T. minuta, which occurred in only one component community and mostly at a lower abundance and prevalence, can be considered satellite species, contributing to the nested structuring pattern and confirming our hypothesis.
Turgida turgida and A. raillieti, which occurred in most of the infracommunities, regardless of the habitat, host gender or season, are widely distributed in Brazil, occurring in all the Brazilian biomes (Santos et al., Reference Santos, Lent and Gomes1990; Vicente et al., Reference Vicente1997; Silva and Costa, Reference Silva and Costa1999). They mostly occur in species of the genus Didelphis, but have also been recorded in other marsupials (Pinto and Gomes, Reference Pinto and Gomes1980; Vicente et al., Reference Vicente, Gomes and Araujo-Filho1982, Reference Vicente1997; Noronha et al., Reference Noronha, Vicente and Pinto2002; Gomes et al., Reference Gomes2003). These helminths, together with C. tentaculata, formed the main core species of the helminth communities of D. aurita. Although there is a great lack of information concerning the ecology of helminths of wild animals, we suggest that these species have wider tolerance limits and niches in relation to their host, as well as to the external environment, when compared to the other helminths found in the present study. These characteristics may result in a larger distribution and abundances throughout the infracommunities and component communities.
Heterostrongylus heterostrongylus also acts as a core species in the present study, however, showing more specific requirements than the previous species, as they were more present in the preserved habitat. This species belongs to the family Angiostrongylidae, most of whose species have heteroxenous life cycles, with molluscs as intermediate hosts, presenting low specificity to infection (Anderson, Reference Anderson2000). Accordingly, preserved environments could provide a greater diversity of resources for the intermediate hosts, contributing to the transmission of this parasite.
Viannaia hamata and T. orloffi also belonged to the core of the helminth communities analysed; however, we suggest they may act as local opportunistic species, as they occurred in high abundance and prevalence, but mostly in the peri-urban environment, in males, and the latter in the dry season. The positive correlation between T. orloffi and V. hamata may be related to similarities in the host acquisition of these parasites, favouring the occurrence of both species in the small intestine. Both species are presumed to have direct life cycles, based on other species of the same superfamily Thrichostrongyloidea and are parasites of Neotropical marsupials (Durette-Desset, Reference Durette-Desset1968; Gomes et al., Reference Gomes2003). In those cases, the host usually becomes infected while feeding on items contaminated with helminth larvae, but the infection can also occur percutaneously (Anderson, Reference Anderson2000). The peri-urban environment provides more food resources for the opossums, where they show higher population densities than in preserved areas (Cáceres and Monteiro-Filho, Reference Cáceres and Monteiro-Filho1998; Gentile et al., Reference Gentile2000; Kajin et al., Reference Kajin2008). Increases in host density may enhance the parasite transmission, especially in directly transmitted parasites (Bordes et al., Reference Bordes2009). In addition, male opossums have larger movements and home ranges than females (Cáceres and Monteiro-Filho, Reference Cáceres and Monteiro-Filho2001), which can increase their chances of coming into contact with parasites. Moreover, the higher abundance and prevalence of T. orloffi observed during dry periods may indicate that rains could carry away the eggs in the soil, reducing its availability to infect hosts.
The helminths G. marsupialis, T. didelphis, R. coronatus and O. microcephalus formed another subset of species on the metacommunity gradient, according to the nestedness. As these helminths occurred in only two kinds of habitats or in lower values than the core species, they are expected to be more specialized than the core ones. Trichuris didelphis was previously recorded only for Didelphis virginiana (Babero, Reference Babero1960) and D. albiventris (Silva and Costa, Reference Silva and Costa1999; Antunes, Reference Antunes2005). Globocephalus marsupialis was previously reported only in Philander frenatus (Freitas and Lent, Reference Freitas and Lent1936), as well as the helminth R. coronatus (Gomes and Vicente, Reference Gomes and Vicente1972). The latter was also reported in D. aurita (Gomes, Reference Gomes1977). Among this subset of helminths, O. microcephalus is the only species that needs an intermediate host, generally invertebrate. Although occurring in all habitats, its prevalence was higher in the peri-urban habitat, like most of the core species, but in female opossums. Invertebrates are common components of the opossum's diet (Santori et al., Reference Santori, Lessa, Astúa and NC2012), which suggests that, in this case, infection may be more related to the diet than to the environmental exposure of the host.
The rare species, such as D. proloba, B. advena and T. minuta, which formed a satellite subset, probably have more specific requirements and narrower niches compared to the other species, as they were present in few infracommunities and in low numbers. Duboisiella proloba has been reported only twice in D. aurita (Travassos et al., Reference Travassos, Freitas and Kohn1969; Gomes, Reference Gomes1977); B. advena has been reported in D. aurita, but more frequently in P. frenatus (Travassos et al., Reference Travassos, Freitas and Kohn1969); whereas T. minuta was reported once in Chironectes minumus, D. albiventris, D. marsupialis, Marmosa (Marmosa) murina and in D. aurita (Noronha et al., Reference Noronha, Vicente and Pinto2002; Antunes, Reference Antunes2005).
Stochastic species loss occurs when species distribution limits are determined by species-specific environmental tolerances (Presley et al., Reference Presley, Higgins and Willig2010), i.e. when each species responds to variations of the environment in a different way. These responses are easier to observe on a larger spatial scale. When analysed separately for each municipality at the infracommunity level, the communities of urban and rural environments presented random patterns, suggesting host–parasite relationships that were specific to each environment. In those cases, the species did not respond to a single gradient, and their occurrences were scattered among the infracommunities. The non-random nested structure in the parasite community of this host became clear only when viewed on the regional scale because D. aurita has a large geographical range, large home ranges and is a generalist, opportunistic species. These characteristics may promote homogenization among individual hosts in terms of their exposure to parasites within a local scale, resulting in a random structure. The checkerboard pattern found for the infracommunities in the sylvatic area, Petrópolis, suggests the possible existence of interspecific competition among the helminths as a metacommunity structuring mechanism (Presley et al., Reference Presley, Higgins and Willig2010). A checkerboard structure may also be attributed to the occurrence of rare species in the communities (Schmera et al., Reference Schmera2018), which mostly occurred in the sylvatic area.
The establishment of each parasite in its host can be attributed to heterogeneity among individual hosts in terms of exposure to parasites (Wilson et al., Reference Wilson and Hudson2001; Poulin, Reference Poulin2013). In addition, extrinsic factors, such as temperature and humidity, can determine the distribution of these parasites, also influencing the survival of some parasite life stages, especially the species whose eggs remain in the soil (Jiménez et al., Reference Jiménez, Catzeflis and Gardner2011, Simões et al., Reference Simões2016). In this way, studies carried out on regional scales, or in a set of ecological communities, may show general ecological patterns such as the structure of a metacommunity (Leibold et al., Reference Leibold2004). In the present study we accepted our hypothesis that the helminth community has a coherent structure on a regional scale, indicating that in this parasite–host relationship the helminth species respond to the environmental gradient non-randomly but gradually and predictably on a wider spatial scale than can be observed at the local level.
Acknowledgements
We would like to thank the staff and students of Laboratório de Biologia e Parasitologia de Mamíferos Silvestres Reservatórios, Laboratório de Hantaviroses e Rickettsioses, Laboratório de Biologia de Tripanossomatíedeos and Laboratório de Pesquisa Clínica em Dermatozoonose from Fiocruz who helped in the field work; Dr Paulo D'Andrea for the ICMBio and INEA licences and for supporting the general project; the coordinator of Fiocruz Atlantic Forest Campus, Gilson Antunes, for providing local facilities and supporting the general project; the team of Fiocruz Atlantic Forest Campus, especially to Marta L. Brandão and José L.P. Cordeiro for local facilities and help in the field work; Marcelo F. Freitas for helping in the field work in all campaigns; Instituto Estadual do Meio Ambiente (INEA) for the collecting licence and for local facilities; Dr R. Cerqueira and Dr P.C. Estrela for the coordination of the PPBio Rede BioM.A project in Petrópolis; and A.P. Gomes for the identification of the Acantocephalan.
Financial support
This project was supported financially by Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq – PPBio Rede BioM.A (457524/2012-0), CNPq – Edital Universal (306352/2014-1), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro-FAPERJ (E-26/111.296/2014), and Instituto Oswaldo Cruz (Fiocruz). SFCN and TSC received grants from CAPES, and RGB from CNPq.
Conflict of interest
None.










