Introduction
Infection with gastrointestinal nematodes is the most important cause of economic losses in small ruminant livestock (Roeber et al., Reference Roeber, Jex and Gasser2013). These parasites have deleterious effects upon the health of animals and their productivity. The control of nematode infection relies almost exclusively on the use of synthetic anthelmintics. However, the high cost of this treatment, parasite resistance to chemical drugs, risk of environmental pollution, and contamination of milk and meat foods are increasing global problems (Adamu et al., Reference Adamu, Naidoo and Eloff2013).
Plants are an important source of bioactive compounds, and interest in the drugs derived from plants has increased worldwide, especially in veterinary parasitology (Irum et al., Reference Irum, Ahmed, Mukhtar, Mushtaq, Mirza, Donskow-Lysoniewska, Qayyum and Simsek2015). As a promising alternative to conventional anthelmintics, the use of botanical products may be an eco-friendly and economically sustainable strategy (Hammond et al., Reference Hammond, Fielding and Bishop1997; Hajaji et al., Reference Hajaji, Alimi, Jabri, Abuseir, Gharbi and Akkari2017).
Members of the Persea genus (Lauraceae) are characterized as having a range of biological activities – antidiabetic (Lima et al., Reference Lima, Vasconcelos, Costa-Silva, Maranhão, Costa, Batista, Carneiro, Soares, Ferreira and Wanderley2012), antidiarrhoeal (Odo, Reference Odo2013), antibacterial (Lu et al., Reference Lu, Chang, Peng, Lin and Chen2012), trypanocidal (Molina-Garza et al., Reference Molina-Garza, Bazaldúa-Rodríguez, Quintanilla-Licea and Galaviz-Silva2014) and pesticidal (Torres et al., Reference Torres, Garbo and Walde2014). Some of these effects have been associated with the lipophilic compounds (e.g. acetogenins and long-chain fatty acid derivatives) that are commonly found in several species of Persea (Lu et al., Reference Lu, Chang, Peng, Lin and Chen2012; Rodríguez-Sánchez et al., Reference Rodríguez-Sánchez, Flores-García, Silva-Platas, Rizzo, Torre-Amione, Peña-Diaz, Hernández-Brenes and García-Rivas2015).
Persea willdenovii is popularly known as bush avocado, pink cinnamon, pau-de-andrade or maracanaúba. There have been no investigations of its biological or chemical proprieties. This study aimed to evaluate the in vitro efficacy of extracts and fractions obtained from the leaves of P. willdenovii on eggs and larvae of gastrointestinal nematodes of goats, and their cytotoxic effects on Vero cells (African green monkey kidney cell line). In addition, gas chromatography–mass spectrometry (GC–MS) analyses were performed to characterize the main constituents present in the most active fraction.
Materials and methods
Plant materials
Leaves of P. willdenovii Kosterm (Lauraceae) were collected during August 2012, in the municipality of Rio de Contas, Bahia, Brazil. Voucher specimens were deposited in the Herbarium of the State University of Feira de Santana, Brazil, with the number 201,559.
Extract preparation
Leaves of P. willdenovii (1325 g) were dried at room temperature, pulverized and macerated with ethanol for 5 days. After filtration, the solvent was removed through evaporation using a rotary evaporator to furnish a crude ethanolic extract (CHE: 482.21 g/36.4%). The CHE was dissolved in an ethanol:water solution (80:20) and subjected to sequential liquid–liquid partition with the following solvents: hexane, ethyl acetate and butanol. The extracts were concentrated individually, yielding: hexane extract (HE: 46.62 g/9.7%), ethyl acetate extract (EAE: 80.19 g/16.6%), butanolic extract (BE: 482.21 g/36.4%) and residual hydroethanol extract (RHE: 85.76 g/17.8%).
Fractionation of the active extract
The most active extract (EAE) against gastrointestinal nematodes of goats was fractionated by column chromatography (CC) in an open column packed with silica gel, eluted with solvents in order of increasing polarity. The fractionation yielded 14 fractions that were analysed by means of thin-layer chromatography (TLC), using a mixture of chloroform and methanol (3:1, v/v) as the mobile phase. The fractions with the same chromatographic profiles were united, resulting in six fractions: Fr1, Fr2, Fr3, Fr4, Fr5 and Fr6.
GC–MS analysis
The active fraction (Fr1) was analysed by GC–MS, performed on a Shimadzu CGMS2010 series gas chromatograph (Shimadzu, Kyoto, Japan) (electron impact ionization 70 eV). Helium was used as the carrier gas at a constant flow rate of 1 ml/min; the general analysis conditions were as follows: RTX-5 capillary column (30 m × 0.25 mm × 0.25 mm, composed of 5% diphenyl–95% dimethyl-siloxane), injector (splitless mode) at 300°C, initial oven temperature at 150°C heated at a rate of 15°C/min; final temperature at 300°C. The mass detector was operated with ionization for electron impact (70 eV) with a scan range from 20 to 700 m/z. The sample was analysed by a 1 μl injection of 10 mg/ml solution in hexane.
Parasitological tests
The eggs and larvae of gastrointestinal nematodes were obtained from faeces collected directly from the rectum of naturally infected goats and were kept at the School of Veterinary Medicine and Animal Science, Federal University of Bahia (EMEVZ-UFBA).
Eggs were recovered as described by Hubert & Kerboeuf (Reference Hubert and Kerboeuf1992) and infecting larvae (L3) were obtained from coproculture according to Ueno & Gonçalves (Reference Ueno and Gonçalves1998). The faecal cultures indicated infection with Haemonchus spp. (80%), Oesophagostomum spp. (17%) and Trichostrongylus spp. (3%).
Egg-hatch assay
This test was performed as described by Coles et al. (Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992). The fresh egg suspension was distributed in 96-multiwell microplates (100 eggs/100 μl per well). In these plates, the extracts and fractions (100 μl) were added in different concentrations for the determination of the EC50 and EC90. The concentrations evaluated were: 2.0, 3.0, 4.4, 6.7 and 10 mg/ml (extracts and Fr2), and 0.03125, 0.0625, 0.125, 0.25 and 0.5 mg/ml (Fr1). The cultures were incubated at 27°C and relative humidity (RH) 80%. A positive control (PC) with thiabendazole (0.025 mg/ml) and a negative control (NC) containing the diluent (1% Tween 80) were performed in parallel. After 48 h, lugol was added and hatched larvae (L1) and eggs were counted in each well. The inhibition percentage of egg hatching was determined using the ratio: [(number of eggs)/(number of eggs + number of L1 larvae)] × 100.
Larval motility assay
For the larval motility assay (Ferreira et al., Reference Ferreira, Castro, Chagas, França and Beleboni2013), a suspension of infective larvae (L3) was distributed in 24-well microplates (50 larvae/100 μl per well) and 100-μl aliquots of the extracts or fractions (10.0 mg/ml) were added to the wells. A negative control (NC) containing Tween 80 (1%) and a positive control (PC) consisting of levamisole (0.5 mg/ml) were prepared After a 24-h incubation at 27°C, the movement of the larvae was stimulated in order to count motile and non-motile larvae. The larvicidal activity was expressed as the percentage of non-motile larvae, which was calculated as the ratio: [(number of non-motile larvae)/(number of motile larvae + number of non-motile larvae)] × 100.
In vitro cytotoxicity tests
Cell line and culture
The Vero cell line (ATCC CCL81) was grown in the Roswell Park Memorial Institute, Buffalo, New York, USA (RPMI) and supplemented with 10% fetal equine serum, penicillin G (100 IU/ml) and streptomycin (100 mg/ml) at 37°C in a carbon dioxide incubator. This cell line was chosen for the cytotoxicity evaluation of P. willdenovii because it is widely used as a screening model for cytotoxicity of plant extracts and other compounds, it is readily available and easy to culture (Bahar et al., Reference Bahar, Deng, Zhu, He, Pandharkar, Drew, Navarro-Vázquez, Anklin, Gil, Doskotch, Werbovetz and Kinghorn2011; Nchu et al., Reference Nchu, Githiori, Mcgaw and Eloff2011).
The cells were distributed into 96-multiwell microplates at a density of 3.5 × 104 cells/well. After a 24-h incubation, the cells were exposed to BE, EAE and its fractions Fr1 and Fr2 diluted in 0.01% dimethylsulphoxide (DMSO). The concentrations tested were 2.0–10 mg/ml (BE, EAE and Fr2) and 0.03125–2.0 mg/ml (Fr1). In the control wells, 0.01% DMSO was used. Cell viability was assessed after 24 h, and the Fr1-treated cells were also evaluated after 48 h.
Cell viability was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and trypan blue exclusion assay.
MTT assay
This test is based on the principle of the conversion of MTT by mitochondrial dehydrogenases of living cells into violet-coloured formazan crystals, the concentration of which can be measured spectrophotometrically (Hansen et al., Reference Hansen, Nielsen and Berg1989). Following cell exposure to the extracts and fractions, the culture medium was removed and then 100 μl of MTT solution in RPMI medium (1 mg/ml) was added. After incubating for 3 h at 37°C in a carbon dioxide incubator, a lysis buffer containing 20% SDS (sodium dodecyl sulphate) and 50% DMF (dimethylformamide) was added to the wells, and plates were incubated at 37°C for another 12 h. The optical absorbance was measured using a wavelength plate reader (492 nm). The results were presented as percentage of cell viability as compared to the control group (considered as 100%).
Trypan blue exclusion assay
After treatment with fractions, the cells were detached from the microplates by exposure to trypsin–ethylenediamine tetraacetic acid (EDTA) solution and then centrifuged. The supernatant was discarded, the cells were resuspended in RPMI and stained with trypan blue (0.1%). An aliquot of the cell suspension was taken in order to count the number of viable and non-viable blue-stained cells in a Neubauer chamber. The results were expressed as percentage of viable cells.
Statistical analysis
For the biological assays, three independent experiments, each including five replicates, were performed. Data were presented as mean values ± standard deviation. The results were subject to one-way analysis of variance (ANOVA) followed by comparison with Tukey's test (5%). The EC50 and EC90 (effective concentrations for 50% and 90% inhibition) for parasitological evaluation, and IC50 (50% inhibitory concentration) for the cytotoxicity test, were calculated using non-linear regression analysis. All statistical analyses were performed using Graph Pad Prism® software, version 5.0 for Windows (GraphPad Software, San Diego, California, USA).
Results
Anthelmintic evaluation
Ovicidal activity
All extracts of P. willdenovii inhibited the egg hatching of gastrointestinal nematodes (P < 0.05), in a concentration-dependent manner. The EC50 values were 2.26, 5.0, 6.9 and 8.1 mg/ml for EAE, BE, CHE and RHE, respectively. Over 90% efficacy was observed only in the treatment with EAE (EC90 = 5.95 mg/ml) and BE (EC90 = 6.6 mg/ml). Moreover, the inhibition percentages of EAE and BE, at a concentration of 10 mg/ml, were no different from that of the positive control (albendazole, 0.025 mg/ml). A weaker ovicidal activity was observed for HE (maximum inhibition: 38%) (fig. 1).

Fig. 1. Mean and standard deviation of the percentage inhibition of hatching of gastrointestinal nematode eggs treated with the extracts obtained from Persea willdenovii leaves. *Significant differences from negative control values (P < 0.05).
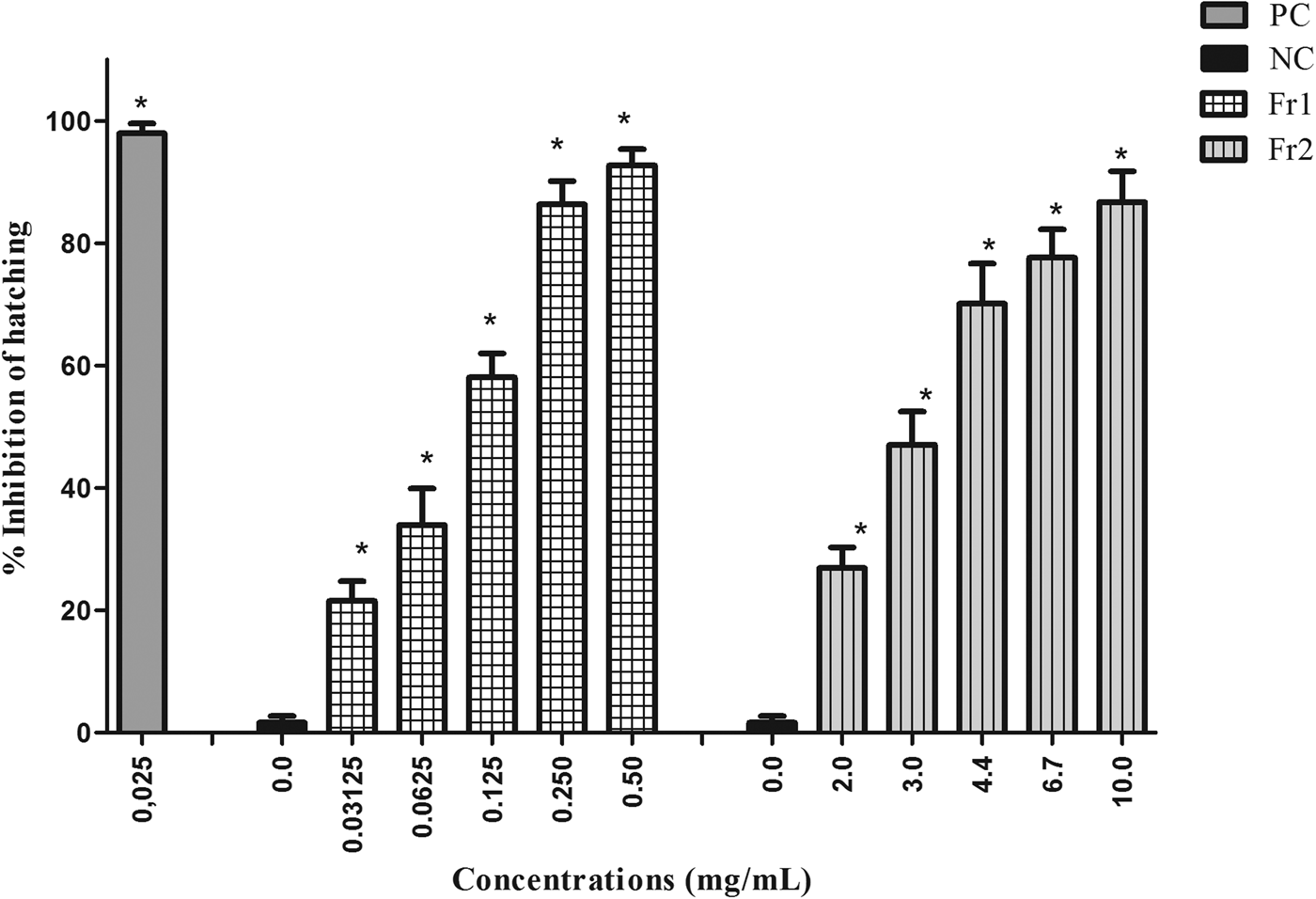
The fractions of the most active extract (EAE) varied in the level of ovicidal activity. Fr3, Fr4, Fr5 and Fr6 exhibited low ovicidal activity. Only Fr1 and Fr2 had a large effect on nematode eggs, with the following EC50 values: 0.12 mg/ml (Fr1) and 2.94 mg/ml (Fr2). Fr1 had an EC90 of 0.29 mg/ml, while Fr2 was not able to inhibit 90% of egg hatching at the concentrations tested (fig. 2).
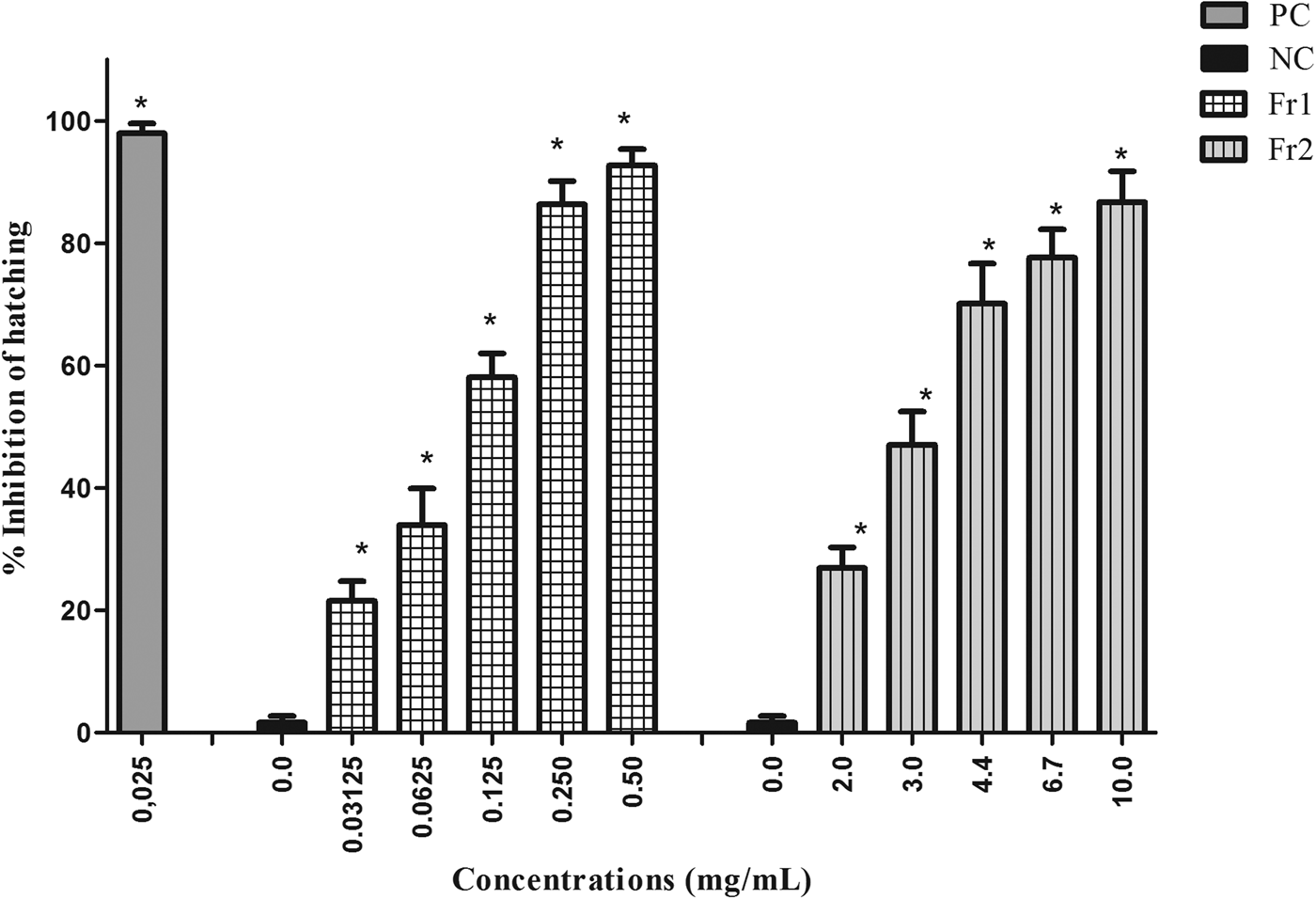
Fig. 2. Mean and standard deviation of the percentage inhibition of hatching of gastrointestinal nematode eggs treated with fractions 1 and 2 (Fr1 and Fr2) of the ethyl acetate extract (EAE) obtained from Persea willdenovii leaves. * Significant differences from negative control values (P < 0.05).
Larvicidal activity
CHE, BE, EAE and RHE exhibited low larvicidal activity, even though the activity differed statistically from the negative control (P < 0.05). HE did not show a significant inhibitory effect on infective larvae. The average efficacy percentages for the CHE (20.4%), HE (10.6%), BE (12.7%), RHE (20.7%) and EAE (34.6%) were much lower than those seen in the positive control group treated with levamisole (99.35%) (fig. 3A).
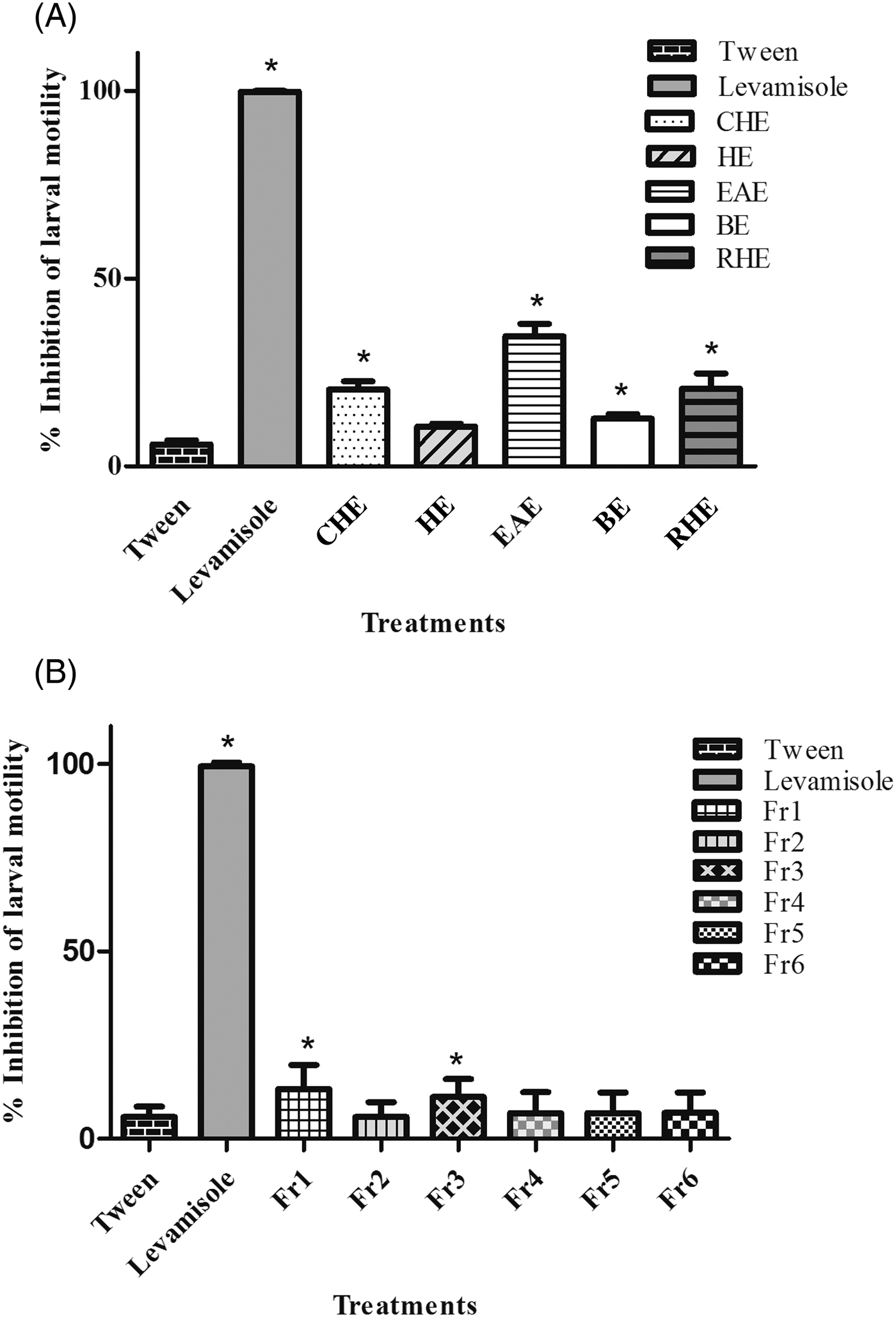
Fig. 3. Mean and standard deviation of the percentage inhibition of motility of L3 larvae of gastrointestinal nematodes after treatment with (A) the extracts (10 mg/ml) and (B) the fractions (10 mg/ml) of the ethyl acetate extract (EAE) obtained from Persea willdenovii leaves. *Significant differences from negative control values (P < 0.05).
The fractions obtained from EAE were also screened for larvicidal activity, and only Fr1 and Fr3 differed significantly from the negative control (P < 0.05) (fig. 3B). The mean percentages of inhibition of larval motility of Fr1 (13.2%) and Fr3 (11.1%) were lower than that exhibited by EAE.
Cytotoxic evaluation
The most active extracts (BE, EAE) and fractions (Fr1, Fr2) were screened for cytotoxicity effects. In the MTT assay, BE, EAE and Fr2 caused a decrease in the viability of Vero cells in a concentration-dependent manner (P < 0.05) after 24 h of treatment. The IC50 values found for BE, EAE and Fr2 were 6.75, 4.95 and 2.66 mg/ml, respectively. Fr2 was more toxic for Vero cells, since there were low cell viability percentages (6–8%) after exposure to this fraction (4.4–10 mg/ml). Fr1 had no significant cytotoxicity on Vero cells at all concentrations tested during 24 h (fig. 4). Moreover, low toxicity was observed in cells treated with Fr1 (0.125–0.5 mg/ml) for 48 h. This longer treatment time induced a reduction of percentage viability (P < 0.05). However, the percentage of viable cells remained above 86% (fig. 5).

Fig. 4. Percentage viability of Vero cells (MTT assay) after treatment for 24 h with (A) the ethyl acetate extract (EAE) and butanolic extract (BE), (B) fraction 1 (Fr1) and (C) fraction 2 (Fr2) of the ethyl acetate extract (EAE), obtained from Persea willdenovii leaves. *Significant differences from negative control values (P < 0.05).
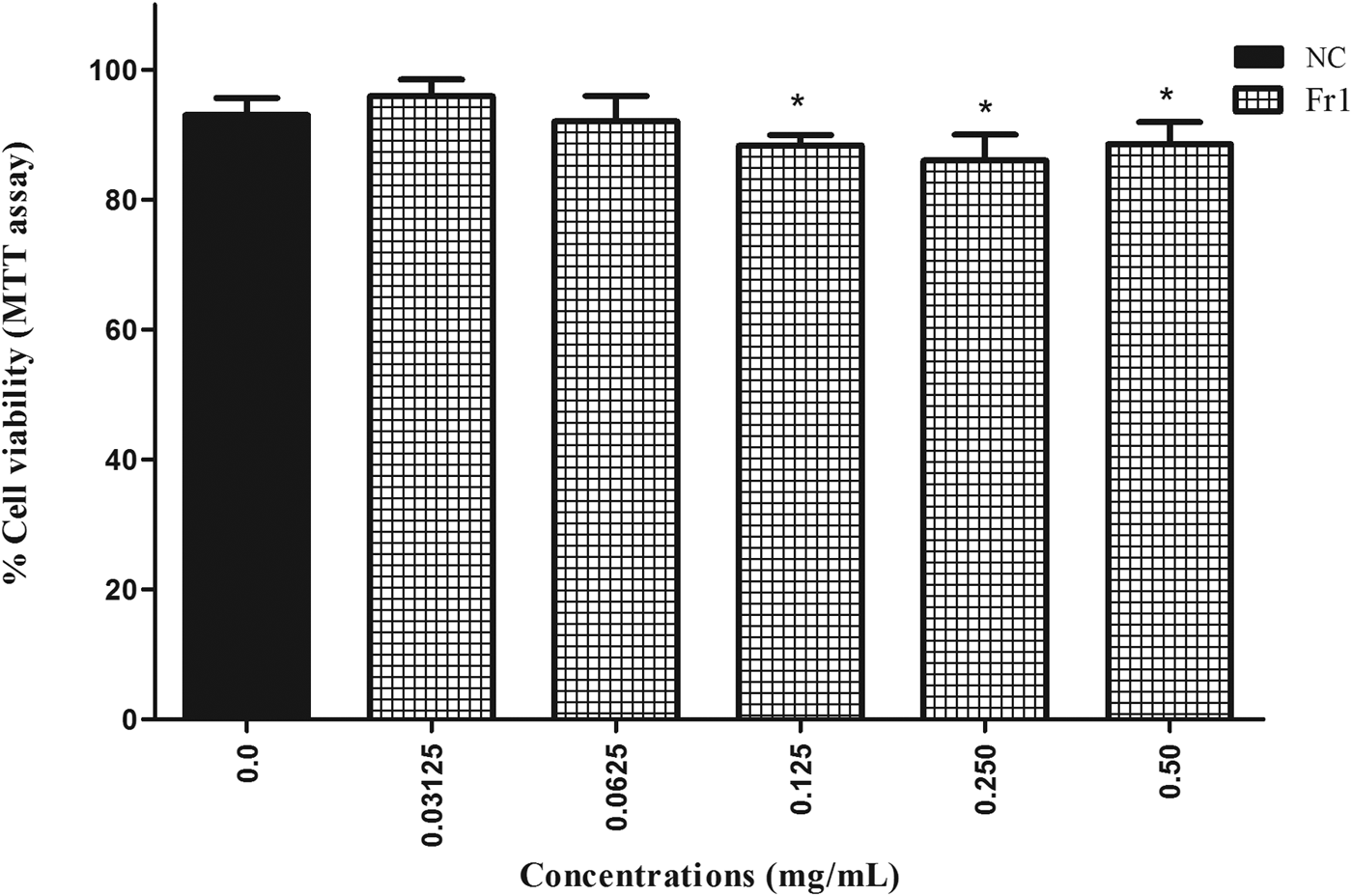
Fig. 5. Percentage viability of Vero cells (MTT assay) after 48 h treatment with fraction 1 (Fr1) of the ethyl acetate extract (EAE) obtained from Persea willdenovii leaves. *Significant differences from negative control values (P < 0.05).
The cytotoxicity of fractions (Fr1 and Fr2) was also investigated using the trypan blue exclusion method. No cytotoxicity was detected with Fr1 treatment, while Fr2 promoted complete loss of cell viability at all concentrations tested. These results were in agreement with the data obtained from the MTT assay.
Chemical analysis
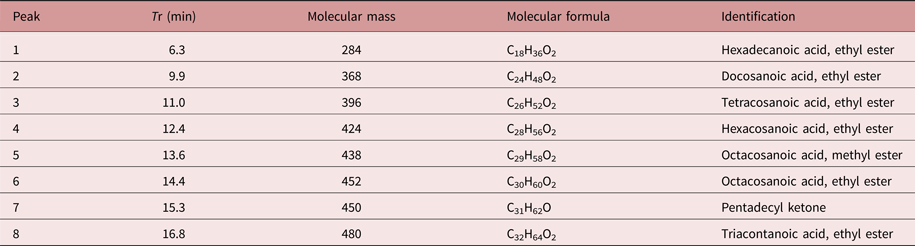
The chemical constituents of the active fraction (Fr1) were analysed by GC–MS. Figure 6 shows the respective total ion chromatogram, which presented eight major peaks eluted between 6 and 17 min. Table 1 shows the MS data and the chemical characterization of each peak. The compounds 1–4, 6 and 8 showed molecular ions of medium intensity and characteristic fragments of ester rearrangement (m/z 88 and 101), being characterized as fatty acid ethyl esters. The compounds 5 and 7 were characterized as a fatty acid methyl ester and a long-chain ketone, respectively.
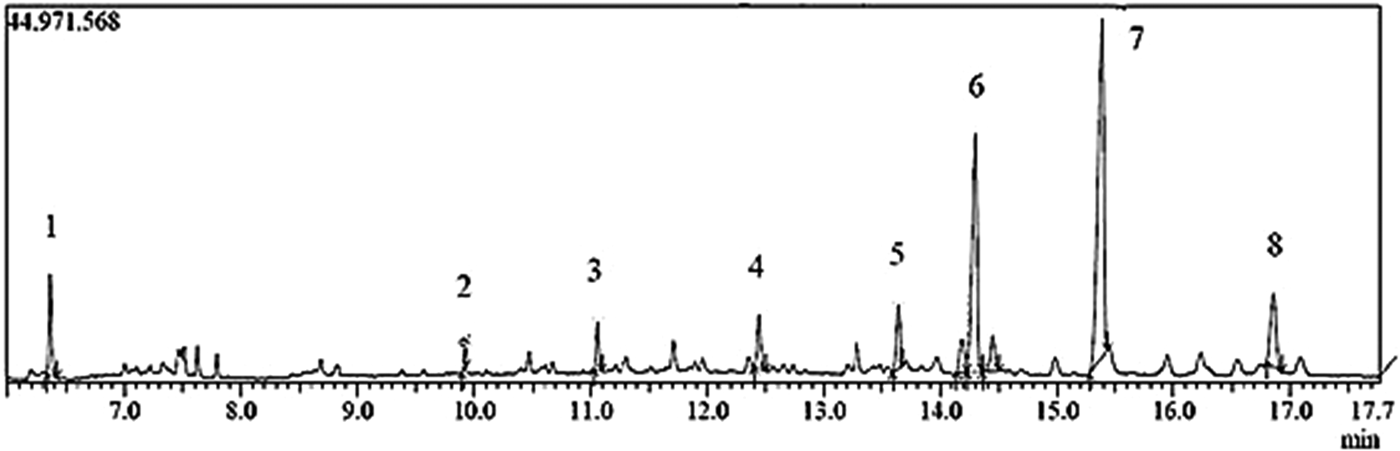
Fig. 6. Chromatogram of total ions (Fr1) obtained from the ethyl acetate extract (EAE) of Persea willdenovii leaves.
Table 1. Chemical characterization of the peaks eluted in the GC–MS chromatogram of the active fraction (Fr1) from Persea willdenovii.
Discussion
Several in vitro assays have been applied in veterinary parasitology to identify plants with anthelmintic activity against different stages of parasites (Grando et al., Reference Grando, de Sa, Baldissera, Oliveira, de Souza, Raffin, Santos, Domingues, Minho, Leal and Monteiro2016). A previous report suggested that a plant extract with EC50 below 6 mg/ml would be a potential candidate as a source of anthelmintic compounds (Adamu et al., Reference Adamu, Naidoo and Eloff2013). Considering this criterion, our results revealed that EAE (EC50 = 2.26 mg/ml) and BE (EC50 = 5.0 mg/ml) showed high ovicidal activity against gastrointestinal nematodes. Hence, the most potent extract (EAE) was fractionated. Fr1 and Fr2 also had a large effect on egg hatching. Fr1 presented the greatest activity, with EC50 and EC90 values approximately 20 times lower than those found for EAE.
The chemical analysis of Fr1 allowed the identification of lipophilic compounds (fatty acid ethyl esters, fatty acid methyl ester and a long-chain ketone). The chemical structures were determined by comparison with data in the literature (Kuo & Shue, Reference Kuo and Shue1991; Jiménez et al., Reference Jiménez, Bernal, Aumente, Nozal, Martín and Bernal2004; Sudha et al., Reference Sudha, Chidambarampillai and Mohan2013; Rushdi et al., Reference Rushdi, Adgaba, Bayaqoob, Al-Khazim, Simoneit, El-Mubarak and Al-Mutlaq2014). The compounds hexacosanoic acid, ethyl ester and pentadecyl ketone had been described previously in other species, such as Cinnamomum reticulatum (Lauraceae) (Kuo & Shue, Reference Kuo and Shue1991), Euphorbia helioscopia L. (Euphorbiaceae) (Nazir et al., Reference Nazir, Ahmad, Naeem and Khan1993), Melia azedarach (Meliaceae) (Ahmed et al., Reference Ahmed, Rao, Ahemad and Ibrahim2012). The latter two species also showed anthelmintic activity against Haemonchus contortus (Maciel et al., Reference Maciel, Morais, Bevilaqua, Camurça-Vasconcelos, Costa and Castro2006; Lone et al., Reference Lone, Chishti, Bhat, Tak and Suhaib2012).
The present work is the first report on the antiparasitic properties and chemical composition of P. willdenovii. The in vitro nematicide effect of another Persea species, P. americana, has been documented. The methanolic extract of the latter plant was active against Bursaphelenchus xylophilus, and this effect was associated with a lipophilic compound, isoobtusilactone A (Dang et al., Reference Dang, Kwon, Choi, Choi, Jang, Park, Lim, Ngoc and Kim2010).
The presence of ethyl esters of long-chain fatty acids, triacontanoic acid and ethyl ester in P. willdenovii may be related to anthelminthic activity. Some fatty acids and their derivatives are considered to be nematicidal phytochemicals (Hernández-Villegas et al., Reference Hernández-Villegas, Borges-Argáeza, Rodríguez-Vivas, Torres-Acosta, Méndez-Gonzáleza and Cáceres-Farfána2012). In vitro and in vivo anthelmintic activity of Phytolacca icosandra against H. contortus was associated with three lipophilic compounds: 2-pentadecanone 6,10,14-trimethyl; pentadecanoic 14-methyl acid, methyl ester; and hexadecanoic acid, ethyl ester (Hernández-Villegas et al., Reference Hernández-Villegas, Borges-Argáeza, Rodríguez-Vivas, Torres-Acosta, Méndez-Gonzáleza and Cáceres-Farfána2011, Reference Hernández-Villegas, Borges-Argáeza, Rodríguez-Vivas, Torres-Acosta, Méndez-Gonzáleza and Cáceres-Farfána2012). Many factors can interfere with nematicidal activity of fatty acids, such as chain length, and number and position of double bonds (Faizi et al., Reference Faizi, Fayyaz, Bano, Iqbal, Siddiqi and Naz2011).
The action of a mixture of five n-alkyl ferulates, including the ferulate triacontyl-compound derivate of triacontanoic acid, on H. contortus was reported by Santos et al. (Reference Santos, López, Meira, Guedes, David, Zacharias, David and Lima2012). These compounds inhibited the parasite proteases, an important group of enzymes participating in the interaction between the helminth and the host.
The mechanisms of action of fatty acids against other micro-organisms (fungi and bacteria) have been suggested to be due to the alteration of membrane fluidity, changes in membrane proteins and cytoplasmic disintegration. Changes in the permeability of membranes may interfere with the helminth egg-hatching process (Rogers & Brooks, Reference Rogers and Brooks1977).
A low in vitro effect on infective larvae (L3) was observed in all extracts and fractions tested. These findings contrast with the high efficacy of EAE and Fr1 on egg hatching. Differences in the sensitivity of eggs and larvae may be associated with differences in enzymatic components and membrane structures of eggs and infective larvae (Mansfield et al., Reference Mansfield, Gamble and Fetterer1992). Previous studies with other plant extracts also reported higher action of P. icosandra and Zizyphus joazeiro extracts against eggs of gastrointestinal nematodes (Hernández-Villegas, Reference Hernández-Villegas, Borges-Argáeza, Rodríguez-Vivas, Torres-Acosta, Méndez-Gonzáleza and Cáceres-Farfána2011; Gomes et al., Reference Gomes, de Lima, Vaz, Santos, Santos, Dias, Botura, Branco and Batatinha2016).
In order to assess the safety of active extracts and fractions, an in vitro cytotoxicity assay was carried out using monkey kidney cells (Vero). The International standard ISO 10993-5 (International Organization for Standardization, 2009) suggested that a substance should be considered toxic when it causes over 30% cellular inviability. According to this parameter, evidence of cytotoxicity was observed in the treatments with EAE, BE and Fr2. Only Fr2 promoted complete cell death in the trypan-blue assay, and the decreasing order of toxicity was Fr2 > EAE > BE. However, Fr1 can be considered to be non-toxic to normal Vero cells at concentrations 16 and 4 times higher than its EC50 value (antiparasitic activity) after 24 and 48 h of treatment in the MTT assay, respectively.
In conclusion, the results of this study revealed an in vitro anthelmintic effect of crude extracts from leaves of P. willdenovii. The ethyl acetate extract showed higher ovicidal activity against gastrointestinal nematodes of goats. The bio-guided separation of fractions demonstrated that Fr1 had the most potent antiparasitic activity and was relatively non-toxic to normal Vero cells. The main compounds of this fraction were fatty acid ethyl esters, a methyl ester fatty acid and a long-chain ketone. Extracts and fractions of P. willdenovii were not effective on the larvae (L3) of these parasites.
To the best of our knowledge, the present work is the first to assess the biological activities and chemical characterization of P. willdenovii, and it contributes to the development of sustainable alternatives to conventional anthelmintics. Additional studies on the complete isolation of active chemical constituents, as well as in vivo tests, are also needed.
Acknowledgements
We thank Abilio Borghi for reviewing the grammar of the manuscript.
Financial support
The authors would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) for financial support.
Conflict of interest
None.
Ethical standards
The procedures performed in this work were approved by the ethics committee for animal use of the School of Veterinary Medicine and Animal Science, Federal University of Bahia (EMEVZ-UFBA) (Protocol 34/2014).