Introduction
Parasites can directly interfere with host individuals, populations, and communities (Poulin Reference Poulin1999; Bellay et al. Reference Bellay, Oda, Campião, Yamada, Takemoto, Dáttilo and Rico-Gray2018), affecting several processes, such as competition, migration, speciation, reproduction, quality of life, and behaviour, and consequently affecting biodiversity (Hudson Reference Hudson, Thomas, Renaud and Guégan2005). In addition, several intrinsic and extrinsic factors may influence the distribution and abundance of parasites, thus modulating parasite diversity (Morand Reference Morand2015). Host attributes, such as host sex and age, have been identified as important factors influencing parasite abundance and prevalence (Costa-Neto et al. Reference Costa-Neto, Cardoso, Boullosa, Maldonado and Gentile2019; Boullosa et al. Reference Boullosa, Cardoso, Costa-Neto, Teixeira, Freitas, Júnior and Gentile2020; Cirino et al. Reference Cirino, Costa-Neto, Maldonado and and Gentile2020).
Helminth community studies of Neotropical marsupials are scarce. Most of them were carried out for large marsupials, such as Didelphis marsupialis (Jiménez et al. Reference Jiménez, Catzeflis and Gardner2011; Freitas et al. Reference Freitas, Maldonado, Mendonça, Ramos, Rossi and Pacheco2022), Didelphis aurita (Costa-Neto et al. Reference Costa-Neto, Cardoso, Boullosa, Maldonado and Gentile2019), Didelphis albiventris (Silva & Costa Reference Silva and Costa1999; Zabott et al. Reference Zabott, Pinto, Viott, Gruchouskei and Bittencourt2017; Cirino et al. Reference Cirino, Costa-Neto, Cardoso, Estrela, Maldonado and and Gentile2022), Metachirus myosurus (Cirino et al. Reference Cirino, Costa-Neto, Maldonado and and Gentile2020), and Philander opossum (Ramírez-Cañas et al. Reference Ramírez-Cañas, George-Nascimento, García-Prieto and Mata-López2019). Much attention has been paid to the species of the genus Didelphis because they can occur in high abundances in peridomicile and rural areas and are known to be reservoirs of zoonoses (Lima et al. Reference Lima, Sarquis, Oliveira, Gomes, Coutinho and Teixeira2012; Jansen et al. Reference Jansen, Xavier and Roque2015; Bezerra-Santos et al. Reference Bezerra-Santos, Fontes, Nogueira, Yamatogi, Ramos, Galhardo, Furtado, Rabelo, Araújo and Campos2020). Nevertheless, for the genus Marmosa, there is only one study comparing the helminth structure of two sympatric species of this genus (M. demerarae and M. murina) in French Guiana (Byles et al. Reference Byles, Catzeflis, Scheibel and and Jiménez2013). The scarcity of such studies concerning small marsupials highlights a large gap in knowledge about this group.
The white-bellied woolly mouse opossum Marmosa constantiae Thomas, 1904 (Didelphimorphia, Didelphidae) is geographically distributed in the central portion of South America and occurs in parts of Brazil, eastern Bolivia, Paraguay, and Argentina (Smith & Owen Reference Smith and Owen2016). In Brazil, it occurs in the northwestern region, including the states of Acre, Amazonas, Mato Grosso, Pará, and Rondônia (Silva et al. Reference Silva, Ferreira and Rossi2019). This species occurs in the Amazon, Cerrado, and Pantanal biomes of the Neotropical region, is arboreal, is often found in the understorey of forests and occasionally at the ground level, and has an insectivorous-omnivorous diet (Faria et al. Reference Faria, Lanes and Bonvicino2019).
There are few studies of helminth species descriptions for M. constantiae (Andrade-Silva et al. Reference Andrade-Silva, Vilela, Freitas, Pacheco, Mendonça, Rossi and Maldonado2022a, Reference Andrade-Silva, Costa, Pacheco, Rossi and Maldonadob). However, there are no studies of its helminth fauna or helminth community structure. The aims of the present study were to describe the species composition and analyse the parasitological parameters of the helminth fauna of M. constantiae in the Amazonian Arc, a municipality of Sinop in the northern state of Mato Grosso, Brazil. We also analysed the helminth community structure of M. constantiae in this locality.
Materials and methods
Study area
This study was carried out on 16 sample transects settled in forest fragments ranging from 81.7 to 19.838 ha in the municipality of Sinop (11°49′1.71″ S, 55°24′39.05″ W) in the state of Mato Grosso, Brazil. The area is within an ecological transition landscape of the Cerrado/Amazon biomes consisting of forest patches surrounded by pastures and monoculture plantations. This area is of special interest not only because it is located between two biomes but because the region of the Amazonian Arc has been suffering strong recent anthropic action (Silva Jr. et al. Reference Silva, Pessôa, Carvalho, Reis, Anderson and and Aragão2021). According to the Köppen classification, the climate in the region is tropical hot and humid (Aw) with monsoon-type rainfall in transition to the superhumid equatorial climate (Am) of the Amazon (Alvares et al. Reference Alvares, Stape, Sentelhas, Gonçalves and Spavorek2013). In this region, the average annual temperatures range from 24 °C to 27 °C, with an average annual rainfall of 2,000 mm. Two well-defined seasons are noted: a rainy season from October to April and a dry season from May to September (Priante-Filho et al. Reference Priante-Filho, Vourlitis, Hayashi, Nogueira, Campelo and Nunes2004).
Sample collection method
Samples were collected during a study of an ectoparasite network of small mammals (Mendonça et al. Reference Mendonça, Colle, Freitas, Martins, Horta and Oliveira2020). Two expeditions were carried out during eight consecutive nights: the first during the rainy season in November–December 2016 and the second during the dry season in June 2017. The marsupials were captured using Tomahawk® (Model 201, 16 in × 5 in × 5 inches, Wisconsin, USA) and Sherman (Model XLK, 3 in × 3.75 in × 12 in, Florida, USA) live traps. Traps were arranged alternately at the ground level and in the understorey (at a height of at least 1.5 m from the ground). The bait was composed of a mixture of banana, peanut butter, cornmeal, sardine, and vanilla flavouring.
Following the standards of the Federal Council of Veterinary Medicine in Resolution No. 1000 (https://www.cfmv.gov.br/) and of the Federal Council of Biology in Resolution No. 301 (https://cfbio.gov.br/), the captured animals were anaesthetized, measured, sexed, and euthanized. All individuals were submitted to taxidermy, and voucher specimens were deposited in the Zoological Collection of the Federal University of Mato Grosso, Cuiabá, state of Mato Grosso, Brazil (Supplementary Table S1).
The study was carried out under approval of the Chico Mendes Institute for Biodiversity Conservation (ICMBio, licence n° 8863-1) for the collection of small nonflying mammals and by the Ethics Committee on the Use of Animals (CEUA) of the Federal University of Mato Grosso (UFMT) (protocol n° 23108.076870/2015-41).
Helminth specimen collection, fixation, and identification
The organs and the gastrointestinal tract of each animal were removed immediately after euthanasia. The stomach, intestines, lungs, liver, and heart of the hosts were separated in Petri dishes and dissected using a stereoscopic microscope for helminth recovery. All helminths found were collected and processed according to Hoffman (Reference Hoffman1987), washed in physiological solution, and stored in 70% ethanol.
Specimens of the phylum Nematoda were cleared with 50% lactophenol, and specimens of the phylum Plathyhelminthes and the phylum Acanthocephala were stained with Carmine of Langeron (Amato et al. Reference Amato, Walter and Amato1991). The specimens were posteriorly placed on temporary slides and examined using a Nikon Eclipse E200MVR light microscope (Nikon Corporation, Tokyo, Japan). Digital images were captured using a compound microscope (Zeiss Standard 20) with TCapture Imaging Application Software Version 5.1.1.0 (N). Specific morphological characters were used to identify the specimens, based on studies by Vicente et al. (Reference Vicente, Rodrigues, Gomes and Pinto1997) and Anderson et al. (Reference Anderson, Chaubaud and Willmott2009), as well as on articles describing related species. The voucher specimens were deposited at the Helminthological Collection of the Oswaldo Cruz Institute (Supplementary Table S1).
Data analysis
The mean abundance, mean intensity, and prevalence of each helminth species were calculated according to Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). We calculated the parasite species richness for each infracommunity; the mean parasite species richness (the mean number of helminth species considering all infracommunities); and the estimated parasite species richness in order to investigate whether the observed number of species was equivalent to the expected number of parasite species for the entire dataset. The estimated species richness was calculated using the nonparametric Jackknife 1 estimator (Magurram Reference Magurram2004).
We compared abundance and prevalence in relation to host sex for helminth species with a prevalence of more than 10%. We used the non-parametric Mann–Whitney test to compare parasite abundance and the χ2 contingency test to compare prevalence. We investigated the influence of host body size on the total abundance (all helminth species) and on species richness considering the infracommunity level using linear regression, separately for each host sex. Regressions were performed between host body size and helminth abundance or richness of each host specimen. The significance of the regression coefficient (beta) was evaluated using a t test. Because body size is related to age, we hypothesized that older hosts would be more parasitized than younger hosts. The analyses were carried out using Past software, version 3.21 (Hammer et al. Reference Hammer, Harper and Ryan2001). We tested the data for normal distribution using the Shapiro–Wilk test. In all analyses, the significance level considered was 5%.
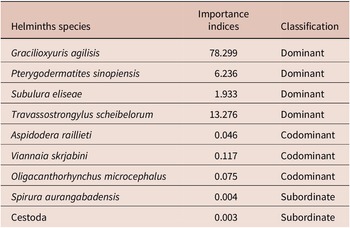
According to Thul et al. (Reference Thul, Forrester and Abercrombie1985), we calculated an importance value I for each of the helminth species. From this, each species was then classified in the community as dominant (I ≥ 1.0), codominant (0.01 ≤ I < 1.0), subordinate (I < 0.01) or an unsuccessful pioneer (I= 0). This analysis takes into account both total abundance and prevalence of each helminth species in relation to the others.
The pattern of the helminth community structure was analysed using the Elements of Metacommunity Structure approach (Presley et al. Reference Presley, Higgins and Willig2010). In the context of the theory of metacommunity, each host containing a community of parasites (i.e., an infracommunity) is considered a local community, and the set of infracommunities forms a metacommunity (i.e., a set of local communities linked by dispersal of multiple species (Leibold et al. Reference Leibold, Holyoak, Mouquet, Amarasekare, Chase and Hoopes2004)). This analysis makes it possible to understand the distribution of parasite species along the environmental gradient and the processes that shape parasite communities. This analysis was done only at the infracommunity level, where each site (local community) in the incidence matrix was represented by a host specimen, as we assessed only one component community. We evaluated the three elements of metacommunity structure (EMS) (coherence, turnover, and boundary clumping) according to the method described by Leibold and Mikkelson (Reference Leibold and Mikkelson2002) and Presley et al. (Reference Presley, Higgins and Willig2010). The coherence element tests whether species respond to the same environmental gradient by quantifying the number of embedded absences, represented by interruptions in the distribution of a species. This element is based on a matrix of incidence of species by sites ordered by reciprocal average. When the coherence element is significant, the other elements are also assessed. The turnover element determines whether the processes that structure the diversity lead to substitution or loss of species along the gradient and is calculated by the number of species replacements in the incidence matrix. Boundary clumping quantifies the overlap of species distribution limits in the environmental gradient, which can be clumped (when the index value is above 1), hyperdispersed (when the index is below 1), or random (when boundary clumping is not statistically significant) (Presley et al. Reference Presley, Higgins and Willig2010; Braga et al. Reference Braga, Oliveira and Cerqueira2017). EMS analysis was performed using the metacom package (Dallas Reference Dallas2020) in R software version 4.0.3 (R CoreTeam 2021). The level of significance was 5% in all the analyses.
Results
Helminth fauna
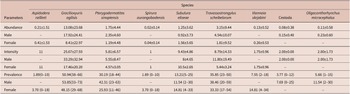
Fifty-three specimens of M. constantiae were captured and analysed – 26 males and 27 females. Among them, 20 specimens were captured during the rainy season and 33 during the dry season. Forty-four animals were parasitized with at least one helminth species. A total of 1,655 helminths were recovered. Seven nematode species were identified: Aspidodera raillieti Travassos, 1913 (Ascaridida: Aspidoderidae); Gracilioxyuris agilisis Feijó et al. Reference Feijó, Torres, Maldonado and and Lanfredi2008 (Oxyurida: Oxyuridae); Travassostrongylus scheibelorum Scheibel et al. Reference Scheibel, Catzeflis and and Jiméñez2014 (Trichostrongylida: Viannaiidae); Viannaia skrjabini Lent & Freitas, Reference Lent and Freitas1937 (Trichostrongylida: Viannaiidae); Spirura aurangabadensis (Ali & Lovekar, Reference Ali and Lovekar1967) (Spirurida: Spiruridae); Subulura eliseae Andrade-Silva et al. Reference Andrade-Silva, Vilela, Freitas, Pacheco, Mendonça, Rossi and Maldonado2022a (Strongylida: Subuluridae); and Pterygodermatites sinopiensis Andrade-Silva et al. Reference Andrade-Silva, Costa, Pacheco, Rossi and Maldonado2022b (Spirurida: Ricticulariidae). The acantocephalan Oligacanthorhynchus microcephalus (Rudolphi, 1819) (Archiacanthocephala, Oligacanthorhynchidae) was also identified. Four specimens of cestode were recovered, but they were not identified at a lower taxonomic level due to the fragmented condition of the material.
The overall helminth species richness was nine, as well as the estimated species richness (Jackknife 1 = 9). The mean helminth species richness was 1.57 and ranged from 0 to 5 in each infracommunity. The highest helminth species richness was observed for a male host, which had five helminth species. We recovered a total of 693 adult helminths of G. agilisis, 167 of T. scheibelorum, 93 of P. sinopiensis, and 66 of S. eliseae, which were the most abundant species (Table 1). The nematodes V. skrjabini, S. auragabadensis, and A. raillieti were found only in female hosts, whereas O. microcephalus and the cestode were found only in male hosts (Table 1).
Table 1. Mean intensity and abundance (± SD) and prevalence rates (with 95% confidence intervals) in relation to host sex for the helminth species found in Marmosa constantiae in Sinop, state of Mato Grosso, Brazil
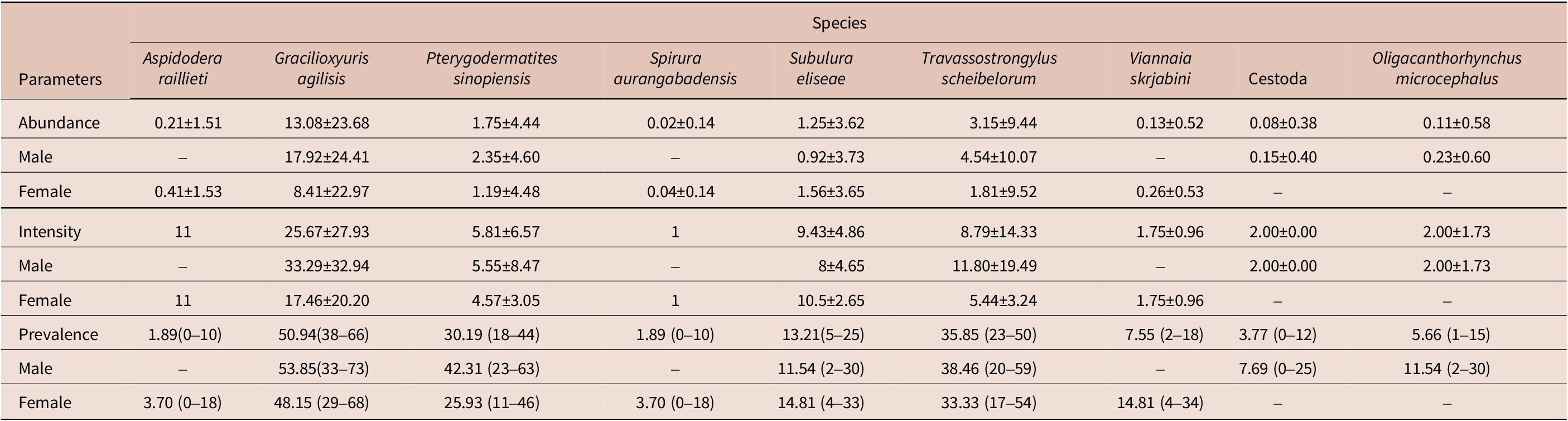
The influence of host sex on helminth abundance and prevalence was investigated for G. agilisis, T. scheibelorum, P. sinopiensis, and S. eliseae. Helminth abundance and prevalence showed no statistically significant differences between host sexes (Tables 2 and 3).
Table 2. Chi-square and probability values of helminth prevalence in relation to host sex for the most abundant helminth species found in Marmosa constantiae in Sinop, state of Mato Grosso, Brazil

Table 3. Mann-Whitney U and probability values of helminth abundance in relation to host sex for the most abundant helminth species found in Marmosa constantiae in Sinop, state of Mato Grosso, Brazil

Concerning the influence of host body size on total helminth abundance or on species richness in each infracommunity, a direct relationship was observed between male host body size and total helminth abundance (beta = 0.416, t = 2.240, p = 0.035). This relationship was not observed in female hosts (beta = 0.077, t = -0.032, p = 0.975). Concerning species richness, no significant relationship was observed for males (beta = 0.141, t = 0.697, p = 0.493) or females (beta = -0.066, t = 0.288, p = 0.776).
The helminth species G. agilisis, T. scheibelorum, P. sinopiensis, and S. eliseae were dominant in the component community of M. constantiae, whereas A. raillieti, V. skrjabini, and O. microcephalus were codominant, and S. aurangabadens and Cestoda were subordinate (Table 4).
Table 4. Importance indices for each helminth species found in Marmosa constantiae in Sinop, state of Mato Grosso, Brazil

The patterns of the helminth metacommunity structure were coherent with more species replacements than species loss, showing a Clementsian pattern (Figure 1). This pattern indicates that species distribution along the environmental gradient was more clumped than expected by chance and that their distribution boundaries were coincident.

Figure. 1 Ordinated matrix, using elements of metacommunity structure (coherence, turnover, and boundary clumping), for the helminth metacommunity of Marmosa constantiae (Didelphimorphia, Didelphidae) in the ecotone area of the biomes Cerrado/Amazonia in Mato Grosso state, Brazil. Abs = embedded absences; p = p-valor (α = 0.05); Mean = mean values of the randomized matrices; SD = standard deviation of the randomized matrices; Rep = number of replacements; MI = Morisita index.
Discussion
Helminth fauna
Here, we provide the first study of the helminth community structure of M. constantiae. This marsupial is a new host of six species of parasites – namely, A. raillieti, G. agilisis, T. scheibelorum, V. skrjabini, S. aurangabadensis, and O. microcephalus. Previous studies based on taxonomic descriptions reported M. constantiae as a type of host for the helminths S. eliseae and P. sinopiensis (Andrade-Silva et al. Reference Andrade-Silva, Vilela, Freitas, Pacheco, Mendonça, Rossi and Maldonado2022a, Reference Andrade-Silva, Costa, Pacheco, Rossi and Maldonadob). The similarity between the observed and the expected helminth species richness indicated that probably no helminth species would be incorporated into the M. constantiae component community with an increase in the number of hosts sampled. The helminth fauna was composed of four helminth species with expected direct life cycles (A. raillieti, G. agilisis, T. scheibelorum, and V. skrjabini) and five species with indirect life cycles (S. aurangabadensis, S. eliseae, P. sinopiensis, O. microcephalus, and Cestoda).
Among the helminth species registered as parasitizing other hosts of the genus Marmosa, Byles et al. (Reference Byles, Catzeflis, Scheibel and and Jiménez2013) reported 12 species for M. demerarae and 14 for M. murina. Among them, only A. raillieti and O. microcephalus were also found parasitizing M. constantiae. However, we observed that five genera were shared among these hosts: Travassostrongylus, Viannaia, Spirura, Subulura and Pterygodermatites.
The study of the helminth fauna of D. marsupialis, which was carried out in the same locality as the present study, reported the same helminth species richness observed for M. constantiae (nine species). However, D. marsupialis and M. constantiae shared only two species, A. raillieti and O. microcephalus, which have been registered in several didelphids (Varella et al. Reference Varella, Vilela, Gentile, Cardoso, Costa-Neto and and Maldonado2022; Freitas et al. Reference Freitas, Maldonado, Mendonça, Ramos, Rossi and Pacheco2022). Jiménez et al. (Reference Jiménez, Catzeflis and Gardner2011) reported 10 helminth species for the marsupial Philander opossum in French Guiana and 12 in Mexico, among which A. raillieti and O. microcephalus were the only species in common with the present study. These results corroborate the generalist characteristics of these two helminth species.
This study provides the first record of the species T. scheibelorum in Brazil and expands its geographic distribution, which has thus far been described only in French Guiana parasitizing M. demerarae and M. murina (Scheibel et al. Reference Scheibel, Catzeflis and and Jiméñez2014). The genus Travassostrongylus has a wide geographical distribution and infects several marsupial species (Vicente et al. Reference Vicente, Rodrigues, Gomes and Pinto1997).
Gracilioxyuris agilisis has already been described as parasitizing other didelphids in the Pantanal and Cerrado biomes (Feijó et al. Reference Feijó, Torres, Maldonado and and Lanfredi2008; Santos-Rondon et al. Reference Santos-Rondon2012). This study is the first report of this helminth in the state of Mato Grosso. The record of this parasite in M. constantiae reinforces the theory that pinworms and didelphids share a close evolutionary history, which corroborates Santos-Rondon et al. (Reference Santos-Rondon2012), including the high prevalence found in both studies (51% and 70%, respectively).
Viannaia skrjabini was reported only in large didelphids, D. aurita and Philander quica (Temminck, 1824) in the state of Rio de Janeiro Brazil by Lent and Freitas (Reference Lent and Freitas1937). The present study expanded the geographical distribution to an ecotone area in the state of Mato Grosso.
Spirura aurangabadensis, in turn, also had its geographic distribution expanded, since it was recorded only in India, parasitizing bats; in Malaysia, parasitizing tree shrews and primates; in Australia, parasitizing bats and marsupials; and in the state of Minas Gerais, Brazil, also parasitizing bats (Ali & Lovekar Reference Ali and Lovekar1966; Quentin & Krishnasamy Reference Quentin and Krishnasamy1975; Spratt Reference Spratt1985, Reference Spratt2007).
Concerning the influence of host sex on parasitological parameters, most studies of mammalian parasites have shown higher rates of infection in male hosts than in females (Zuk & McKean Reference Zuk and McKean1996; Poulin Reference Poulin2007). However, no significant differences were observed in the abundance and prevalence of helminths between male and female hosts in our study. Regarding host body size, male host body size was an important variable for the abundance of helminth species. In studies carried out with didelphids, this relationship between host body size and helminth abundance has already been demonstrated, postulating that larger and older hosts have more parasites than young hosts (Cirino et al. Reference Cirino, Costa-Neto, Maldonado and and Gentile2020; Freitas et al. Reference Freitas, Maldonado, Mendonça, Ramos, Rossi and Pacheco2022) because they may acquire parasites during their lifetime.
Helminth community structure
Most helminth species were considered dominant or codominant in the study. Only S. aurangabadensis and the cestode species were considered subordinate, which indicates that these species occur infrequently and do not contribute significantly to the community (Thul et al. Reference Thul, Forrester and Abercrombie1985). Indeed, as previously mentioned, S. aurangabadensis may be associated with bat fauna and may represent an occasional record of M. constantiae infection. The cestode, in turn, had low prevalence and the lowest abundance in the study.
The Clementsian pattern of metacommunity structure recorded for infracommunities indicates that the distribution of the helminths in M. constantiae is more clustered than expected by chance, forming compartments of species that replace each other along the environmental gradient – in this case, among infracommunities. Each compartment tends to be formed by species that present ecological or biological similarities or by species with interdependent phylogenetic relationships, indicating that clusters between infracommunities were formed by parasite species that co-occurred in the same infracommunity, according to the metacommunity theory (Leibold & Mikkelson Reference Leibold and Mikkelson2002). Each host–parasite interaction is a result of coevolutionary processes related to the encounter–compatibility filters (Combes Reference Combes2001) between parasites and hosts. Furthermore, among the dominant species, only G. agilisis and P. sinopiensis were widely dispersed across the hosts, suggesting that they are core species of the helminth fauna of M. constantiae. These species showed high prevalence in the metacommunity.
The Clementsian pattern has been observed in other studies of helminth metacommunity structure. Dallas and Presley (Reference Dallas and Presley2014) reported this pattern in the helminth metacommunity of rodents in New Mexico, which was associated with a high parasite specificity. Costa et al. (Reference Costa, Cardoso, Costa-Neto, Alvarez, Maldonado and Gentile2022) recorded a quasi-Clementsian pattern – analogous to the Clementsian pattern – for helminths of sigmodontine rodents in an agroforestry mosaic in the Brazilian Atlantic Forest. For Brazilian marsupials, there are only two studies of helminth metacommunity structure, and both are for the genus Didelphis (Cirino et al. Reference Cirino, Costa-Neto, Cardoso, Estrela, Maldonado and and Gentile2022; Costa-Neto et al. Reference Costa-Neto, Cardoso, Boullosa, Maldonado and Gentile2019). Cirino et al. (Reference Cirino, Costa-Neto, Cardoso, Estrela, Maldonado and and Gentile2022) recorded a Gleasonian pattern for helminths of Didelphis albiventris in two extremes of the Atlantic Forest. Although Gleasonian and Clementsian patterns present distinct characteristics regarding the processes that promote such distributions, both are characterized by a greater species replacement than species loss along an environmental gradient. In contrast, Costa-Neto et al. (Reference Costa-Neto, Cardoso, Boullosa, Maldonado and Gentile2019) observed a quasi-nested structure with more species loss than species replacement for D. aurita in peri-urban, sylvatic, and rural environments in southeastern Brazil.
Our results indicate that host sex was not related to abundance or prevalence. However, male host body size influenced both parameters. The pattern of the community structure considering the infracommunities in this locality indicates more species replacement than species loss along the environmental gradient. This is an unprecedented study of the helminths of M. constantiae in an ecotone area between Cerrado and Amazonia that contributes to filling gaps in the knowledge of the helminth fauna of neotropical marsupials and to understanding the patterns of parasite community structure.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0022149X2300038X.
Acknowledgements
We are grateful to Leodil da Costa Freitas for the excellent field work to collect marsupial hosts and screen parasitic helminths. We thank the Universidade Federal Mato Grosso for providing the helminths used in this study.
Authors’ contribution
BEAS, AMJ, and RG contributed to the study conception and design. RFBM, RVR, and RCP carried out the fieldwork and collected samples. BEAS, TSC, and RG analysed the data. The first draft of the manuscript was written by BEAS, AMJ, and RG. All authors contributed to the final version of the manuscript. All authors read and approved the final manuscript.
Financial support
This study was financed in part by funding a scholarship as provided by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - Brasil – Finance code 001, Fundação de Amparo à Pesquisa do Estado de Mato Grosso - FAPEMAT (#477017/2011), and Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (#447557/2014-9; #310352/2016). RG received grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro - FAPERJ (E-26/010.001597/2019).
Competing interest
The authors declare no competing interests.
Ethical standard
This study was approved by the Animal Use Ethics Committee (CEUA) - UFMT under protocol number 23108.076870/2015 and the Chico Mendes Institute for Biodiversity Conservation (ICMBio) under protocol number 8863-1.