Introduction
Helminths are an important component of natural ecosystems, where they exert effects on their hosts’ growth, physical performance, fecundity and population sizes, which affect connections throughout the ecological network (Thomas et al., Reference Thomas, Poulin, de Meeüs, Guégan and Renaud1999). Helminth infracommunities in amphibians are typically less diverse than those in birds or mammals, but they contain relatively high numbers of helminths that are core species in several hosts (Aho, Reference Aho, Esch, Bush and Aho1990). Amphibians are placed in the middle of food webs, being important prey items for many vertebrates and major predators of invertebrates (Duellman & Trueb, Reference Duellman and Trueb1994), which determines their role as intermediate, definitive or paratenic hosts for many parasites with complex life cycles (Smyth & Smyth, Reference Smyth and Smyth1980; Galaktionov & Dobrovolskij, Reference Galaktionov and Dobrovolskij2003; Toledo & Fried, Reference Toledo, Schwartz, Nomura, Aguiar, Velota, da Silva and Anjos2019).
There are many studies on helminth community compositions in amphibian hosts across Europe. Relatively recent works have been published concerning post-metamorphic ranid frogs from Germany (Andreas, Reference Andreas2006) and Poland (Popiolek et al., Reference Popiolek, Rozenblut-Koscisty, Kot, Nosal and Ogielska2011; Okulewicz et al., Reference Okulewicz, Hildebrand, Łysowski, Bunkovska and Perec-Matysiak2014), bufonid toads from Belarus (Shimalov & Shimalov, Reference Shimalov and Shimalov2001) and Pelophylax spp. frogs from Serbia (Bjelić-Čabrilo et al., Reference Bjelić-Čabrilo, Popović and Paunovic2009) and Ukraine (Kuzmin et al., Reference Kuzmin, Dmytrieva, Marushchak, Morozov-Leonov, Oskyrko and Nekrasova2020). Annotated helminth check lists are available for the main post-metamorphic anuran hosts (Rana spp., Bufo bufo, Pelophylax spp.) from the Volga River basin of Russia (Chikhlyaev & Ruchin, Reference Chikhlyaev and Ruchin2014, Reference Chikhlyaev and Ruchin2021; Chikhlyaev et al., Reference Chikhlyaev, Kirillova and Kirillov2018a, Reference Chikhlyaev, Ruchin and Fayzulinb, Reference Chikhlyaev, Ruchin and Fayzulin2019a, Reference Chikhlyaev, Ruchin and Fayzulinb, Reference Chikhlyaev, Ruchin and Kirillov2020) and the Ural Region of Russia (Burakova & Vershinin, Reference Burakova and Vershinin2016; Burakova & Baytimirova, Reference Burakova and Baytimirova2017; Vershinin et al., Reference Vences, Galan, Palanca, Vieites, Ni Eto and Rey2017). Brief reports on helminthological investigations in post-metamorphic newts have been made for Greece (Sattaman, Reference Sattaman1990) and Belarus (Shimalov et al., Reference Shimalov, Shimalov and Shimalov2001), and recently the Lissotriton vulgaris helminth community composition has also been studied by combining morphological identification and DNA sequencing (Sinsch et al., Reference Sinsch, Heneberg, Těšínský, Balczun and Scheid2019). Older studies, often published as brief notes in local sources in national languages, have also been summarized (Ryzhikov et al., Reference Ryzhikov, Sharpilo and Shevechenko1980; Sudarikov et al., Reference Sudarikov, Shigin, Kurochkin, Lomakin, Stenko and Yurlova2002). However, helminth community studies in European amphibian larval stages are very few (Avery, Reference Avery1971), in contrast with many studies in North America (e.g. Bursey & Dewolf, Reference Bursey and Dewolf1998; Koprivnikar et al., Reference Koprivnikar, Baker and Forbes2006; Rhoden & Bolek, Reference Rhoden and Bolek2015).
A number of local or intrinsic factors affect helminth aggregations in amphibians, such as spatial heterogeneity, host body size, parasite dimensions and virulence, and parasite and intermediate-host productivities (Campião et al., Reference Campião, Ribas, Morais, Silva and Tavares2015; Johnson & Wilber, Reference Johnson and Wilber2017; Toledo et al., Reference Toledo and Fried2017; Mihaljevic et al., Reference Mihaljevic, Hoye and Johnson2018). In addition, fish studies suggest a high importance of individual host susceptibility (Tinsley et al., Reference Tinsley, Rose Vineer, Grainger-Wood and Morgan2020) and show more complicated mechanisms determining helminth infection rates, such as ontogenic dietary shifts that change parasite intake pathways (Saad-Fares & Combes, Reference Saad-Fares and Combes1992), or the presence of certain parasite infection levels that are optimal for the host body condition (Maceda-Veiga et al., Reference Maceda-Veiga, Green, Poulin and Lagrue2016). Common invasion pathways or biological interactions (such as mutual facilitation or competition) may cause parasite species associations in hosts (Dallas et al., Reference Dallas, Laine and Ovaskainen2019) that, so far, have been noted in very few amphibian helminth community studies (Hamann et al., Reference Hamann, González and Kehr2006a, Reference Burakova and Baytimirova2010, Reference Hamann, Kehr and González2013a, Reference Hamann, Kehr and González2014). Many studies have shown the effect of anthropogenic habitats, such as soybean agriculture (Koprivnikar & Redfern, Reference Koprivnikar and Redfern2012), pasture and rice agriculture (Hamann et al., Reference Hamann, González and Kehr2006b, Reference Hamann, González and Fernández2020; Campião et al., Reference Campião, Ribas, Silva, Dalazen and Tavares2017), crop vs. livestock land uses (Draghi et al., Reference Draghi, Drago, Saibene and Agostini2020; Portela et al., Reference Portela, Dos Santos and Dos Anjos2020), and urban and pesticide polluted areas (King et al., Reference King, McLaughlin, Gendron, Pauli, Giroux, Rondeau, Boily, Juneau and Marcogliese2007), on their composition, species richness and abundances. Helminths may provide clues to freshwater trophic state and water quality (Zargar et al., Reference Yang, Harlow, Puggioni and Redding2011), but, in general, the effect of freshwater habitat qualities on helminth communities in vertebrate hosts remains understudied. Thus, to our knowledge, there are no studies on the effect of waterbody size, which clearly affects habitat spatial diversity and waterbody buffering capacities against external pressures (Biggs et al., Reference Biggs, von Fumetti and Kelly-Quinn2016) that could be important for complex trematode life cycles (Galaktionov & Dobrovolskij, Reference Galaktionov and Dobrovolskij2003).
Surprisingly few studies in Western Palearctic amphibians have paid attention to species associations, relationship with host size or the habitat effect other than simple terrestrial vs. aquatic division. Some of these surveys confirm species richness or parasite total abundances as being higher in larger hosts (Andreas, Reference Andreas2006; Kuzmin et al., Reference Kuzmin, Dmytrieva, Marushchak, Morozov-Leonov, Oskyrko and Nekrasova2020), while others indicate the prevalence and intensity being positively related to the host size in separate trematode species (Abdel-Gaber et al., Reference Abdel-Gaber, Maher, Kamel and El Deeb2017; Ozoliņa et al., Reference Ozoliņa, Deksne, Pupins, Gravele, Gavarane and Kirjušina2021) or describe changes in the post-metamorphic ranid frog infracommunities along the urbanization gradient (Vershinin et al., Reference Vences, Galan, Palanca, Vieites, Ni Eto and Rey2017). Antagonism between some nematode and trematode species has been noted in communities in the Danube basin (Bjelić-Čabrilo et al., Reference Bjelić-Čabrilo, Popović and Paunovic2009).
Amphibian helminth communities in Latvia and the east Baltic region were virtually unstudied before the present survey, with the single exception being a study on Alaria alata mesocercariae infections in ranid and bufonid amphibian hosts (Ozoliņa et al., Reference Ozoliņa, Deksne, Pupins, Gravele, Gavarane and Kirjušina2021). Since 2016, amphibians have been targeted by several state-wide monitoring surveys in Latvia, which allowed us to visit many sites and perform extensive sampling for parasitological investigations. For the present study, we sampled amphibians from different taxonomical (anurans from two families and newts), ontogenic (larval and post-metamorphic) and ecological (terrestrial, semi-aquatic, aquatic) groups. The aims of this study were: (1) to provide basic information on helminth infracommunities in Latvian amphibians (such as species lists and standard parasitological parameters); (2) to compare helminth communities between hosts from various taxonomical, ecological and ontogenic groups; (3) to identify species associations; and (4) to find out helminth infection relationships with host ontogeny (stage, size) and waterbody size, which could be related to ecological traits or life cycles of both hosts and parasites.
Materials and methods
Study sites and sampling
Amphibians were collected in June–August of 2017–2020 at 174 sites covering the whole territory of Latvia (fig. 1). We sampled seven hosts from six amphibian taxa: 370 post-metamorphic water frogs (a species complex, Pelophylax spp.) from 107 sites (range 1–26; median 2 per site); 90 water frog tadpoles from ten sites (1–70; 2.5); three moor frogs (Rana arvalis) from two sites; 26 common frogs (Rana temporaria) from seven sites (1–16; 1); 53 common toads (B. bufo) from 22 sites (1–18; 1); 249 larval smooth newts (L. vulgaris) from 53 sites (1–22; 2.5); and 18 larval great crested newts (Triturus cristatus) from 13 sites (1–2; 1). The water frog (Pelophylax) species complex was identified only to a generic level, because the species separation in a typical for Latvia mixed lessonae-esculentus populations produce many errors when based solely on morphological data (Mayer et al., Reference Mayer, Hawlitschek, Zahn and Glaw2013), but some other study showed a lack of substantial effect of water frog genetics on helminth communities (Popiolek et al., Reference Popiolek, Rozenblut-Koscisty, Kot, Nosal and Ogielska2011).

Fig. 1. Location of sampled sites.
Water frogs, their tadpoles and newt larvae were collected by hand net in waterbodies, while terrestrial amphibians (Rana, Bufo post-metamorphs) were collected by hand. A sampler spent ~ 20 min at each site, typically collecting all available amphibians, except T. cristatus, which is a rare and protected species in Latvia and, therefore, its samples were limited to 1–2 specimens per site, depending on observed abundance. Amphibians were placed into separate plastic boxes with water and aeration holes and transported to the laboratory. Post-metamorphic amphibians were measured and weighed. Their size ranges covered the variation in the whole population: Pelophylax spp. 2.1–11.1 (median 5.4); R. temporaria 1.8–9.2 (5.2); R. arvalis 2.9–6.4 (5.3); and B. bufo 2.0–10.1 (6.8) cm.
Helminth investigations
Parasitological investigations of collected animals were carried out during the 24 h after samples were delivered to the laboratory (Daugavpils University Ethical committee decision no. 26/2). The animals were euthanized in accordance with Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes and according to the guidelines of the Federation of European Laboratory Animal Science Associations (FELASA) (Guillen, Reference Guillen2012), by a percussive blow to the head under the supervision of a FELASA Category C certified specialist. A full standard parasitological investigation of the euthanized animals was carried out (Skryabin, Reference Skryabin1928; Justine et al., Reference Justine, Briand and Bray2012), including examination of skin that was peeled off and rinsed in distilled water and all internal organs, body cavity, visceral membranes and limb musculature that were dissected, compressed between two slides and examined with the aid of microscopy. Encapsulated larval stages were released from surrounding tissues and analysed at ×100–400 magnification. A total of 841 host specimens were analysed. Helminth identification was based solely on their morphology. They were identified, mainly to species level, with the aid of essential references containing taxonomic keys and species descriptions (e.g. Ryzhikov et al., Reference Ryzhikov, Sharpilo and Shevechenko1980; Sudarikov et al., Reference Sudarikov, Shigin, Kurochkin, Lomakin, Stenko and Yurlova2002; Jones et al., Reference Jones, Bray and Gibson2005).
Data analyses
The role of each helminth taxon in the community was described by a standard set of parameters used in parasitology (Bush et al., Reference Bush, Lafferty, Lotz and Shostak1997), such as abundance (A), prevalence (P) and infection intensity (I). For host-specific infracommunity descriptions, we used species richness (S), natural logarithm Shannon–Wiener diversity index (H′) and Pielou evenness (J′) (Shannon & Weaver, Reference Shannon and Weaver1949; Pielou, Reference Pielou1966). We chose the Sorensen index (Magurran, Reference Magurran2004) for species composition (or qualitative) comparison due to the small relative biases caused by undersampling (Chao et al., Reference Chao, Chazdon, Colwell and Shen2006), and the Morisita–Horn index (Horn, Reference Horn1966) for comparisons of helminth proportions in infracommunities (or quantitative comparison), due to its suitability for surveys with unequal sampling (Chao et al., Reference Chao, Chazdon, Colwell and Shen2006). We performed Kendall's rank correlation (KR) to identify helminth associations in hosts. In each helminth species, we also estimated the host-specific dominance index, which was calculated as the abundance ratio to the abundance of the most numerous species (Poulin et al., Reference Poulin, Luque, Guilhaumon and Mouillot2008). We performed linear discriminant analysis (LDA) to dimensionally reduce infracommunity data and visualize their placement along canonical axes. In the LDA, we used a data set with samples containing at least two parasite species (sample sizes: Pelophylax spp. post-metamorphs 203; Pelophylax spp. tadpoles 32; B. bufo 44; R. temporaria 15; R. arvalis 2; L. vulgaris 32; T. cristatus 2).
We performed zero-inflated Poisson regression (ZIP) and zero-inflated negative binomial regression (ZINB) with a constant inflation option to detect statistically significant helminth infection relationships with host size or waterbody area. While both these regression types are used to deal with data sets with excessive zeroes, ZINB is generally a recommended option for overdispersed data distributions (Yang et al., Reference Vershinin, Burakova and Vershinina2017), which was typical for our helminth counts. However, ZIP models may actually perform better on empirical overdispersed count data (Zell et al., Reference Zargar, Yousuf, Chishti, Ahmed, Bashir and Ahmed2019) and, therefore, we used both ZIP and ZINB in our analyses. The independent unit in our parasite–host size analyses was a sample, but in the parasite–waterbody size analyses it was a site. We pooled data from both hosts’ sexes because their separate analyses produced basically the same results but strongly reduced data pool, and several studies have confirmed the absence of effect of sex on helminth prevalence and intensities in given host taxa (Andreas, Reference Andreas2006; Abdel-Gaber et al., Reference Abdel-Gaber, Maher, Kamel and El Deeb2017; Ozoliņa et al., Reference Ozoliņa, Deksne, Pupins, Gravele, Gavarane and Kirjušina2021).
In our parasite response to host size analysis, we created a series of host and parasite taxon-specific data sets, where we placed only the samples from sites where a given helminth taxon was detected in a given host. For statistically significant models, we referred to McFadden's pseudo R 2 as a measure of goodness of fit for non-nested models (Menard, Reference Menard2000) and the z-score as a measure of data dispersion in the correlations (Sprinthall, Reference Sprinthall2011) to evaluate which host size parameter – length (cm) or weight (g) – better fitted the data. Populations of the same amphibian species can vary across sites in length–weight response curves. This could be caused by site-specific food resource availability, habitat thermal properties, sex, population genetics, etc. (Duellman & Trueb, Reference Duellman and Trueb1994) that may blur a parasite–host size relationship when the pooled data from several sites are being used. However, it may cause only Type II errors (showing a poorer relationship than actually occurs), which cannot invalidate the detected overall (or typical) trend in a given parasite–host system.
In the parasite response to waterbody size analysis, we created host-specific data sets where helminth average abundances per site, site average and total species richness were dependent variables and the waterbody area (ha) was a predictor. This analysis was limited to two hosts (Pelophylax spp. post-metamorphs and L. vulgaris larvae) and to sites with at least three samples per given host present. Here, we also omitted sites where samples were collected in unclosed linear habitats (channels, ditches or riverbanks) that did not allow meaningful area measurements. Waterbody areas were measured on orthophoto maps using the Google Earth Pro software (Google LLC, Mountain View, California, USA) and ranged from 0.01 to 16.92 (median 0.09) ha.
The possible host size relationship with the waterbody size was tested by the Poisson regression (PR). The correlation was absent (dependent variable: host length (cm); predictor: waterbody area (ha); log likelihood ratio chi-square = 1.25, McFadden's pseudo R 2 = 0.001, likelihood ratio chi-square test P = 0.264).
LDA was performed on Past 4 (developed by Ø. Hammer, D.A.T. Harper and P.D. Ryan from the Natural History Museum, University of Oslo, Norway). KR, PR, ZIP and ZINB were performed on STATA 14.2 (StataCorp LLC, College Station, Texas, USA) with the Stata Technical Bulletin insertion ‘Scalar measures of fit for regression models’ (developed by J. Scott Long, Indian University and Jeremy Freese, University of Wisconsin-Madison, USA).
Results
The full results of the parasitological investigation are given in supplementary tables S1–S5. We identified a total of 17 trematode, one monogenean, seven nematode and one acanthocephalan species in our samples (table 1). The richest were the infracommunities in post-metamorphic water frogs (Pelophylax spp.), which contained 25 species. The poorest were larval T. cristatus infracommunities, with only four species, and the most skewed were also communities in amphibian larval stages (Pelophylax spp., L. vulgaris). Dominant or sub-dominant helminth species in Latvian amphibians were trematodes – A. alata (in R. temporaria, Pelophylax spp.), Diplodiscus subclavatus (in R. arvalis), Opisthioglyphe ranae (in Pelophylax spp., L. vulgaris) and Tylodelphys excavata (in Pelophylax spp.), and nematodes – Hedruris androphora (in T. cristatus), Neoraillietnema praeputiale, Oswaldocruzia filiformis and Rhabdias bufonis (all in B. bufo, R. temporaria) (table 2).
Table 1. Helminth infracommunities in amphibians from Latvia: abundance (A; average for all samples ± standard deviation), prevalence (P, %), species richness (S; total and average for infected samples ± standard deviation), diversity (H′) and evenness (J′) in major taxonomic groups and life stages.

a Unidentified taxa. larv, larvae; ad, adults; n.e., not estimable.
Table 2. Dominance index for helminth taxa in post-metamorphic (PM) and larval (L) amphibian hosts.
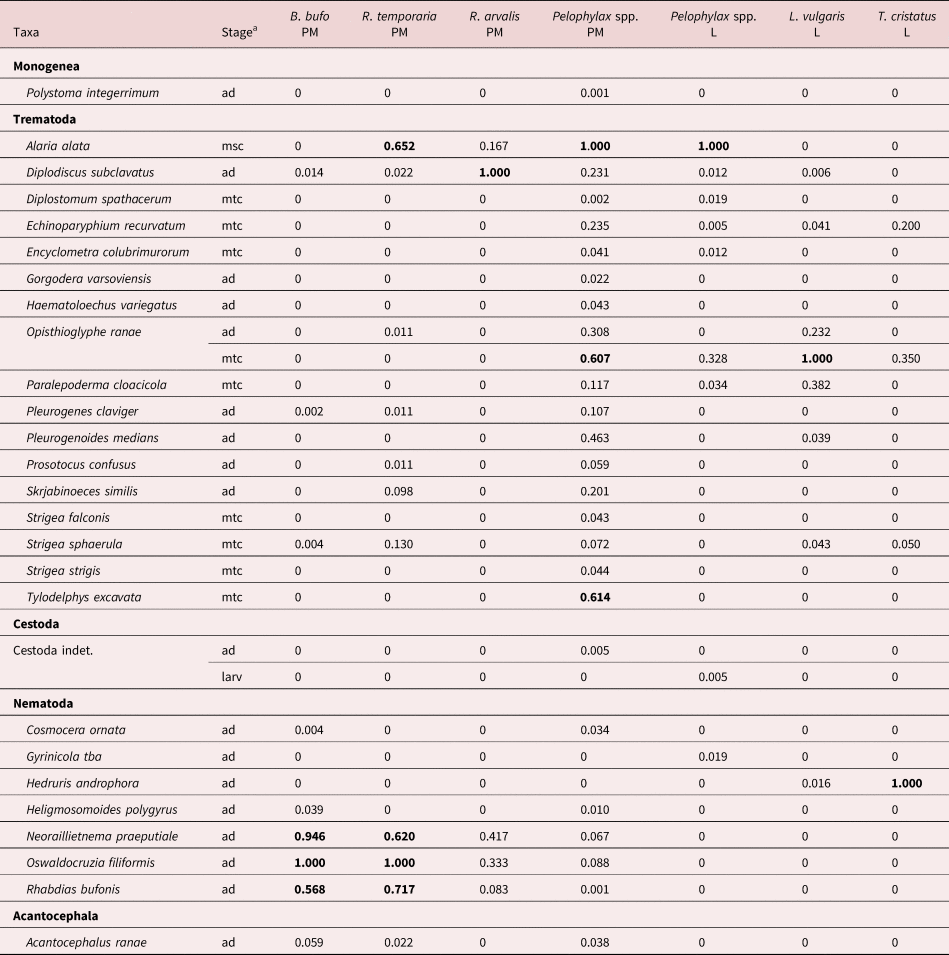
a msc – mesocercariae, mtc – metacercariae, larv – larvae, ad – adults. Index values >0.5 in bold.
Helminth infracommunity similarities depended on both host taxonomic closeness and common habitat (table 3). A terrestrial Ranidae frog (R. temporaria) yielded infracommunities that quantitatively (in species proportions) were more similar to those of a terrestrial Bufonidae toad (B. bufo), while qualitatively (in species composition alone) they had about equal similarities to both a Bufonidae toad from the same habitat and a Ranidae frog (Pelophylax spp.) from a different habitat. In semi-aquatic Ranidae frog (Pelophylax spp.) post-metamorphs, the helminth species composition was more similar to that in the terrestrial Ranidae post-metamorphs, while quantitatively their communities were more similar to those in their own larval stages. In amphibian larval stages, infracommunities in the first newt species (L. vulgaris) were more similar to those in frog tadpoles than those in the other newt species in both qualitative and quantitative indices, while the opposite was true in the second newt species (T. cristatus).
Table 3. Infracommunity comparisons between hosts by Sorensen index for qualitative (upper-right section) and Morisita–Horn index for quantitative (lower-left section) similarities (R. arvalis skipped due to small sample size).

In the LDA, which we used to discriminate infracommunities from various hosts, the first two canonical axes explained 92.4% of the total variation (fig. 2). The first axis (eigenvalue 1.82, 78.6% of variation) alone was the main discriminant function, which arranged hosts along the aquatic–terrestrial habitat gradient, with the highest positive loadings being from dominant terrestrial nematodes, R. bufonis (0.243) and N. praeputiale (0.159), but negative from a rare trematode (Diplostomum spathacerum −0.332), an acantocephalan (−0.180), a monogenean (−0.374) and aquatic nematodes (H. androphora −0.287; Gyrinicola tba −0.235; Heligmosomoides polygyrus −0.156; Cosmocera ornata −0.146). All trematode species with a dominance index >0.1 and the dominant nematode, O. filiformis, had loadings within the interval between −0.05 and +0.05. The second axis was far less important (eigenvalue 0.32, 13.8% of variation), and separated hosts within the aquatic ecosystem, with the highest positive loading coming from the nematode H. androphora (4.373) but negative loadings from some other aquatic nematodes (G. tba −0.294; C. ornata −0.267), a monogenean (−0.373) and some trematodes (Haematoloechus variegatus −0.171; Skrjabinoeces similis −0.138). Hence, the LDA analysis separated larval great crested newt (T. cristatus) infra-communities from those in other aquatic and semi-aquatic hosts, mainly by the dominance of the aquatic nematode, H. androphora, but the dominance of terrestrial nematodes (R. bufonis, N. praeputiale) distinguished terrestrial amphibian (B. bufo, R. temporaria) infracommunities.
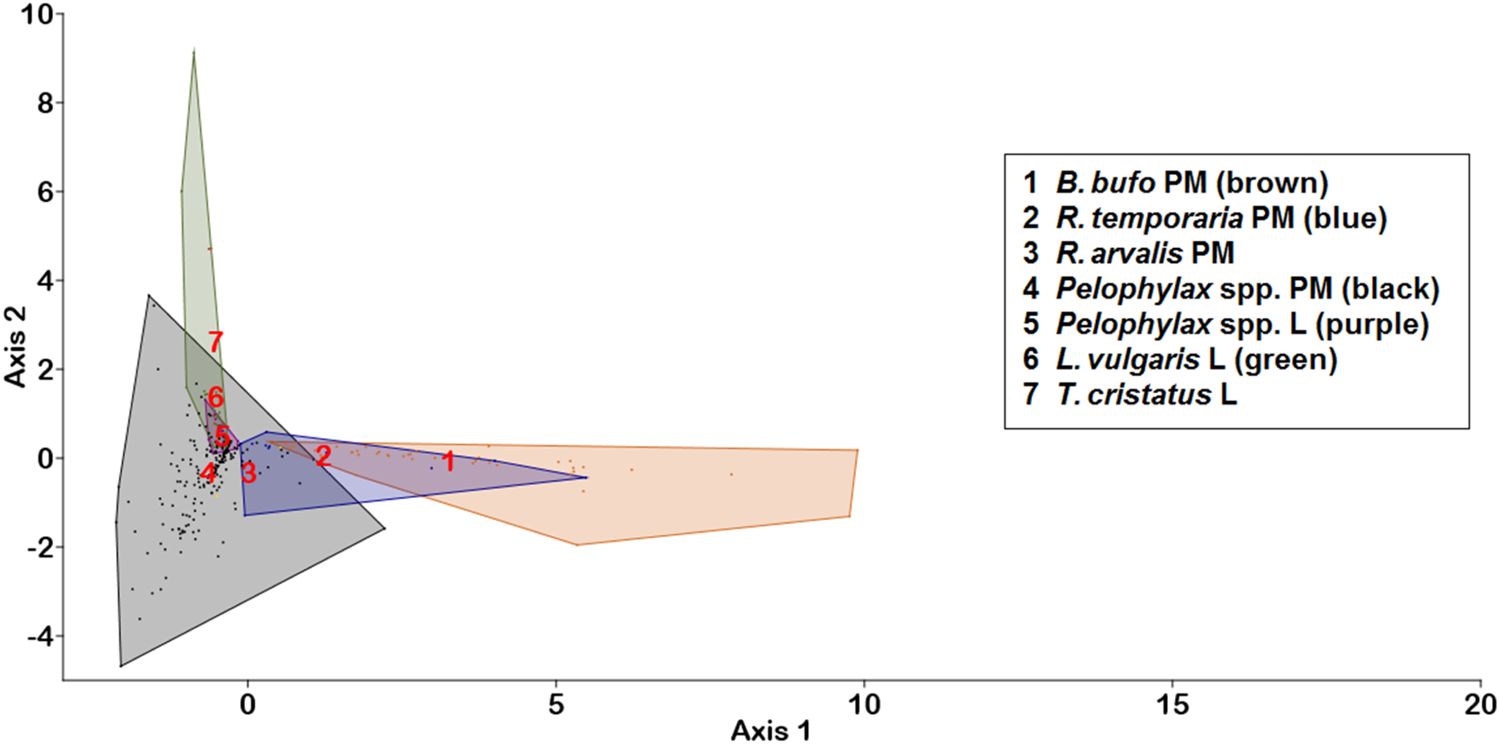
Fig. 2. LDA chart for helminth infracommunities in post-metamorphic (PM) and larval (L) hosts. Numbers denoting the host taxa are placed on group means; hosts with less than three samples have no convex hulls in the chart. Axis 1 can be interpreted as an aquatic–terrestrial habitat gradient, while axis 2 separates hosts in the aquatic habitat.
The Kendall rank correlation matrix for helminth species associations is given in supplementary table S6. All but one (nematode H. androphora) helminth species were correlated to the other species (on average, 7.3 associations per species), where their positive associations (present in 96% of species) were far more typical than negative ones (present in 36% of species), and, with the single exception of Paralepoderma cloacicola, positive associations were also statistically stronger (fig. 3). The most involved were adult plagiorchiid trematodes, which always had associations with other species from their own guild, diplostomid larval trematodes and an acanthocephalan. Gastrointestinal nematodes showed notable difference in aquatic vs. terrestrial infection route species since aquatic C. ornata possessed an association pattern similar to that of the plagiorchiid trematodes, while terrestrial N. praeputiale and O. filiformis were associated mainly with other terrestrial nematodes. A markedly negative association was found between the lung nematode, R. bufonis, and the plagiorchiid larvae guild.

Fig. 3. The strengths of species associations within helminth infra-communities given as the summations of the statistically significant (P < 0.05) tau-b statistics in the Kendall's rank correlation matrix (number of associations in parentheses).
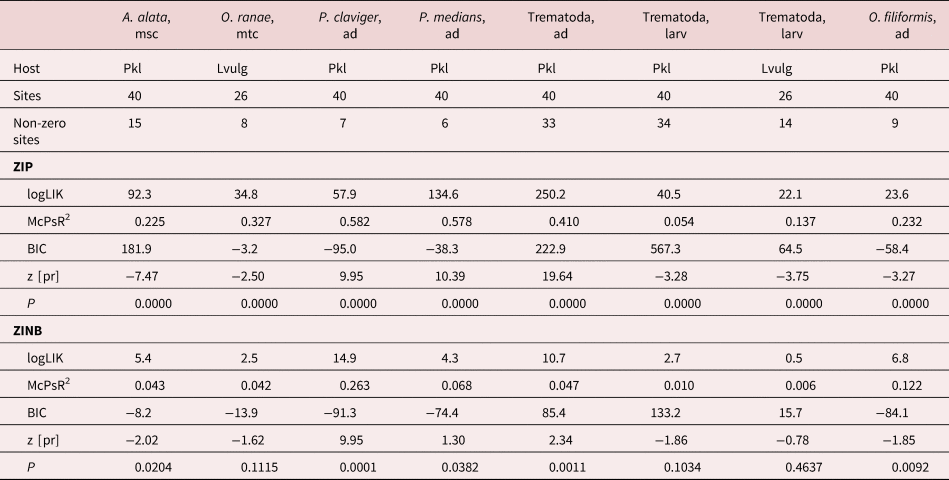
Of the 22 helminth infection–host length models, ZIP performed better in 18 cases, as indicated by the Bayesian information criterion (BIC), but in two cases the models were equal (table 4). In two more cases, ZINB had slightly higher BIC values, but ZINB and ZIP had equal, or the latter had slightly higher, pseudo R 2 values. Hence, ZIP was clearly preferable over ZINB. Both helminth abundances and species richness had positive relationships with the host size. In adult trematode abundances, there was a better relationship with host weight; while in adult trematode species richness, larval trematode and nematode abundances and species richness relationships were better with host length (table 5). Relationships with host size were also present in six trematode and three nematode species. Larval trematode, A. alata, and gastrointestinal nematodes N. praeputiale and O. filiformis infections were better correlated to host length, while adult trematode – Pleurogenes claviger – to the host weight. Two other trematode species (Echinoparyphium recurvatum, P. medians) and the lung nematode, R. bufonis, had a similar goodness of fit in both length and weight models. Two larval trematode species (Strigea strigis, T. excavata) had a negative relationship with host size.
Table 4. Summary statistics for zero-inflated Poisson regression (ZIP) and zero-inflated negative binomial regression (ZINB) models for helminth abundance (A) or species richness (S) relationships with host length and in post-metamorphic amphibian hosts.

a Gastrointestinal nematodes, all nematode species of our study except R. bufonis. logLIK, log likelihood ratio chi-square; McPsR2, McFadden's pseudo R 2; BIC, Bayesian information criterion; P, likelihood ratio chi-square test.
Table 5. Comparison of goodness of fit and dispersion in zero-inflated Poisson regression (ZIP) models for helminth abundances (A) or species richness (S) relationships with host length vs. host weight.

Only statistically significant relationships given. Hosts: Pkl, Pelophylax species complex; RT, Rana temporaria; BB, Bufo bufo; McPsR2, McFadden's pseudo R 2; z [pr], z-score for the predictor.
a Pooled for all the species except R. bufonis; likelihood ratio chi-square test P < 0.01 in all the models.
Of eight helminth infection–waterbody size models, ZIP was better in seven cases, but in one case BIC was slightly higher in ZINB (table 6). Species richness was not related to waterbody area, but a positive relationship was found in pooled adult trematode abundance and in two separate adult trematode species (P. claviger, P. medians). A negative correlation with waterbody size was found in pooled larval trematode abundance in two hosts, in two separate larval trematode species (A. alata, O. ranae) and in the nematode, O. filiformis.
Table 6. Summary statistics for zero-inflated Poisson regression (ZIP) and zero-inflated negative binomial regression (ZINB) models for helminth abundance relationships with waterbody area in semi-aquatic and aquatic amphibian hosts.

Pkl, post-metamorphic Pelophylax spp.; Lvulg, Lissotriton vulgaris larvae; logLIK, log likelihood ratio chi-square; McPsR2, McFadden's pseudo R 2; BIC, Bayesian information criterion; P, likelihood ratio chi-square test; z [pr], z-score for the predictor.
Discussion
The results of our study fit general patterns with semi-aquatic amphibians yielding the richest helminth communities (Aho, Reference Aho, Esch, Bush and Aho1990), nematodes dominating in terrestrial amphibian taxa and trematodes in semi-aquatic taxa (Hamann et al., Reference Hamann, Kehr and González2013b; Okulewicz et al., Reference Okulewicz, Hildebrand, Łysowski, Bunkovska and Perec-Matysiak2014; Burakova & Vershinin, Reference Burakova and Vershinin2016). The water frogs (Pelophylax spp.) at our study sites were the core hosts, with rich trematode communities of both larval and adult stages with diverse life cycles and ecological connections (fig. 4). Nematode species richness was similar in several hosts, but their loads peaked in terrestrial B. bufo. Amphibian larval stages basically yielded a depauperated community of semi-aquatic frogs, supplemented by some rare nematode species (G. tba, H. androphora). The admission of H. androphora separated larval newt, especially T. cristatus, communities from other aquatic and semi-aquatic hosts. It is noteworthy that the post-metamorphic newts from the neighbouring Belarus had infracommunities very similar to those of Pelophylax spp. frogs in our study (Shimalov et al., Reference Shimalov, Shimalov and Shimalov2001), while they were very different in more distant Germany (Sinsch et al., Reference Sinsch, Heneberg, Těšínský, Balczun and Scheid2019). There were two dominant trematode species in Latvia – A. alata and O. ranae – that infected both larval and post-metamorphic amphibian stages. Unlike O. ranae, A. alata had a statistically significant positive relationship with host size that may look like a steady accumulation over host ontogeny. However, plotting of prevalence and infection loads against ontogenetic stage and host size classes showed a sharp decline in prevalence in the first post-metamorphic size (fig. 5), which could be caused by the elevated mortality of infected tadpoles around the metamorphosis stage. In larval O. ranae infections, such a decline was absent.

Fig. 4. Structure of helminth infracommunities in amphibian hosts from Latvia (R. arvalis omitted due to small sample size). Typical helminth species from each unit given on top of their columns. x-axis: first row = taxonomic group; second row = life stage in amphibians (trematodes; from aquatic habitat) or parasite larval habitat (nematodes); third row = other hosts (trematodes) or location in hosts (nematodes). Abbreviations: AQUA, aquatic; OPT, Odonata, Plecoptera, Ephemeroptera, Trichoptera; Gen arthr, wide range of arthropod hosts.

Fig. 5. Prevalence (P, %; dashed line) and infection intensities (I, aver; columns) in two dominant trematode species larval stages over water frog (Pelophylax spp.) host ontogeny. x-axis = ontogenic stage and post-metamorphs’ size class; left y-axis = prevalence; right y-axis = infection intensity (data from two A. alata-infected and five O. ranae-infected sites). Abbreviations: msc, mesocercariae; mtc, metacercariae; aver, average.
Opisthioglyphe ranae was also a dominant or common species in studies in Germany (Andreas, Reference Andreas2006), Poland (Okulewicz et al., Reference Okulewicz, Hildebrand, Łysowski, Bunkovska and Perec-Matysiak2014), Ukraine (Kuzmin et al., Reference Kuzmin, Dmytrieva, Marushchak, Morozov-Leonov, Oskyrko and Nekrasova2020), Serbia (Bjelić-Čabrilo et al., Reference Bjelić-Čabrilo, Popović and Paunovic2009), Russia's Volga River (Chikhlyaev et al., Reference Chikhlyaev, Kirillova and Kirillov2018a) and the Ural region (Vershinin et al., Reference Vences, Galan, Palanca, Vieites, Ni Eto and Rey2017), where A. alata was absent or rare. The lack of A. alata at many of these sites could be explained by habitat type since our survey showed its preference for smaller waterbodies, but waterbodies were large, or their type was not specified in the studies above. One other study showed A. alata domination in frogs in some smaller pools behind beaver dams (Chikhlyaev & Ruchin, Reference Chikhlyaev and Ruchin2020). Alaria alata preferences toward particular amphibian host species may also vary across sites. While its abundance and prevalence were higher in Pelophylax frogs than in Rana (represented mainly by R. temporaria) frogs in our study, the opposite was true for France (Patrelle et al., Reference Patrelle, Portier, Jouet, Delorme and Ferte2015) and Germany (Andreas, Reference Andreas2006). In Russia, the highest loads have been registered in R. arvalis, while Pelophylax spp. and R. temporaria were much less infected (Chikhlyaev & Ruchin, Reference Chikhlyaev and Ruchin2020). Alaria alata may be present at low loads in the common toad (B. bufo), while the natterjack toad (Epidalea calamita) that breeds exclusively in small, very shallow pools (Drobenkov, Reference Drobenkov2015), can be extremely heavily infected, reaching 50% prevalence and loaded with up to 1600 larvae per single host (Shimalov & Shimalov, Reference Shimalov and Shimalov2001).
Trematode infracommunities in post-metamorphic amphibians from Latvia were more variable than those from sub-tropical Argentina (Hamann et al., Reference Hamann, Kehr and González2013b): while semi-aquatic Pelophylax spp. yielded more species in total (but not on average per host specimen) and had more even (as indicated by Pielou's J′) trematode communities, terrestrial B. bufo and R. temporaria yielded poorer and typically more skewed communities compared with the Argentinian ones. Nematode-dominated communities in terrestrial anurans from Brazilian rainforest had lower species richness and were more variable in diversity and evenness than those from Latvia (Toledo et al., Reference Toledo and Fried2017). Most of the helminth species of our study were involved in associations with other helminths. Dominant species typically had many associations, with the only exceptions being T. excavata and H. androphora. Latvian infracommunities yielded much more associations per parasite species (7.3 on average) than those in Argentina (0.1–0.3 on average), where negative associations were equal to or more frequent than positive ones (Hamann et al., Reference Hamann, González and Kehr2006b, Reference Hamann, Kehr and González2010, Reference Hamann, Kehr and González2013a). In Latvia, positive associations dominated, which is consistent with the data from small mammals (Dallas et al., Reference Dallas, Laine and Ovaskainen2019), suggesting that this could be a more typical pattern in small tetrapod communities. Negative associations between soil-transmitted nematodes O. filiformis, N. praeputiale, R. bufonis and many trematodes (especially larval O. ranae and P. cloacicola) of our study could be explained by terrestrial vs. aquatic infection routes. However, in trematodes, we did not find evidence for the intra-guild association dominance over inter-guild ones and causes for species associations in most cases are unclear and require further study.
Helminth infracommunity structure could be a good indicator of its host's habitat and feeding habit preferences, showing differences in a microhabitat or food object selection not detected by their ecology studies. In our study, a terrestrial frog, R. temporaria, yielded a transitional community between that of a semi-aquatic frog (Pelophylax spp.) and a terrestrial toad (B. bufo), as indicated by its depauperate trematode infracommunities and intermediate nematode loads (fig. 4). Rana temporaria is an explosive breeder, present in waterbodies only in early spring, to a lesser extent than B. bufo (Čeirāns et al., Reference Čeirāns, Pupina and Pupins2020), but it has more water-permeable skin (Bentley & Yorio, Reference Bentley and Yorio1976) and may move to shoreline habitats during the summertime (Vences et al., Reference Tyler2000), where trematodes may ascend with occasional aquatic prey. The other Rana species in our study – R. arvalis – had only three samples but they were in line with other studies (Andreas, Reference Andreas2006; Okulewicz et al., Reference Okulewicz, Hildebrand, Łysowski, Bunkovska and Perec-Matysiak2014; Vershinin et al., Reference Vences, Galan, Palanca, Vieites, Ni Eto and Rey2017; Chikhlyaev & Ruchin, Reference Chikhlyaev and Ruchin2020) that showed trematode infection rates in R. arvalis being higher than those of R. temporaria. Together, this suggests an increase of terrestriality in the following order: Pelophylax spp. – R. arvalis – R. temporaria – B. bufo, whereas the last three hosts are usually regarded as equally terrestrial in their ecology accounts (e.g. Arnold & Ovenden, Reference Arnold and Ovenden2002; Speybroeck et al., Reference Speybroeck, Beukema, Bok, Van and Velikov2016).
The nematode R. bufonis was virtually absent in Pelophylax spp. frogs, but it was frequent in R. temporaria frogs, and especially in B. bufo toads. Rhabdias bufonis differs from other nematodes of our study in being a lung parasite, which invades the amphibian host through skin penetration by the larvae produced by free-living generation in soil, where they arise from hosts’ faeces (Spieler & Schierenberg, Reference Spieler and Schierenberg1995). Its free-living form has an extremely short life-span (Gems, Reference Gems and Lee2002), and it is highly unlikely that terrestrial amphibians could attain the 50% prevalence observed in this nematode (Okulewicz et al., Reference Okulewicz, Hildebrand, Łysowski, Bunkovska and Perec-Matysiak2014) from contact with random soil. Since amphibians prefer shelters with moist soil (Cohen & Alford, Reference Cohen and Alford1996), which facilitate nematode survival (Coleman & Wall, Reference Coleman, Wall and Paul2015), we suggest that the host will be infested mainly in regularly used terrestrial shelters where they defecate, but host-specific R. bufonis infection rates could be caused by differences in host fidelities in the use of such shelters.
The higher overall infestation of larger hosts in our study was in line with other studies (Andreas, Reference Andreas2006; Abdel-Gaber et al., Reference Abdel-Gaber, Maher, Kamel and El Deeb2017; Toledo et al., Reference Toledo and Fried2017; Kuzmin et al., Reference Kuzmin, Dmytrieva, Marushchak, Morozov-Leonov, Oskyrko and Nekrasova2020) and could be caused either by higher parasite intake rates by larger hosts or by the accumulation of parasites with host age (Poulin, Reference Poulin2007). Our data suggest the presence of both mechanisms in the studied infracommunities: in intermediate-host generalist plagiorchiid trematodes, P. claviger and P. medians, which have a short-living adult stage in frogs (Sudarikov et al., Reference Sudarikov, Shigin, Kurochkin, Lomakin, Stenko and Yurlova2002), higher infection rates in larger frogs could be attained by an increase of prey intake volumes over the ontogeny, while in A. alata mesocercaria, a similar trend could be caused by a larger frog's ability to consume infected prey – for example, tadpoles (Pearson, Reference Pearson1956) – and subsequent accumulation of a long-living parasite stage (Möhl et al., Reference Möhl, Grosse, Hamedy, Wüste, Kabelitz and Lücker2009).
In amphibians, disease or infection is usually accompanied by emaciation and weight loss (Bancila et al., Reference Bancila, Hartel, Plaiasu, Smets and Cogalniceanu2010). Diplostomid trematode (A. alata, S. strigis) larvae and gastrointestinal nematode infections were better correlated with host length than with weight, probably due to their adverse effect on host body condition (Hendrikx & Van Moppes, Reference Hendrikx and Van Moppes1983; Koprivnikar et al., Reference Koprivnikar, Marcogliese, Rohr, Orlofske, Raffel and Johnson2012; Svinin et al., Reference Svinin, Bashinskiy and Litvinchuk2020) and accompanying weight loss that increased data dispersion. A similar pattern was also observed in trematode species richness. Adult trematode infections, however, were better correlated with host weight. This could be explained by higher parasite intake rates with food by active and healthy hosts, and lower pathogenicity of the adult stages (Koprivnikar et al., Reference Koprivnikar, Marcogliese, Rohr, Orlofske, Raffel and Johnson2012) that did not significantly affect host body conditions.
Post-metamorphic amphibians are considered to have a generally low susceptibility to trematode cercaria infections compared to tadpoles (Koprivnikar et al., Reference Koprivnikar, Marcogliese, Rohr, Orlofske, Raffel and Johnson2012). However, with few exceptions (A. alata, D. spathacerum, O. ranae), larval trematodes in our study were less abundant in tadpoles, several diplostomid species were missing and larval trematodes, as a group, increased in larger hosts in both species richness and sheer abundances, indicating a substantial intake by post-metamorphic frogs. Their intake pathways were not always clear. Thus, we found a trend towards infection increase over host ontogeny in the echinostome, E. recurvatum metacercaria. Although echinostome infections are well studied in larval amphibians (e.g. Holland, Reference Holland2009; Orlofske et al., Reference Orlofske, Belden and Hopkins2013; Goren et al., Reference Goren, Routtu and Ben-Ami2014), this particular species was extremely rare in our tadpole samples and we did not find a description of its infection routes into post-metamorphic frogs. The typical second intermediate host for this species are regarded to be snails and not amphibians (Huffman & Fried, Reference Huffman and Fried2012). Larvae predilection sites in frogs in our study (fig. 6) may give some clues – for example, E. recurvatum metacercaria were located mainly around the eyes, which suggests that the main penetration route for the cercaria could be through the eye mucosa, while a significant proportion of metacercaria of some other taxa (Encyclometra colubrimurorum, P. cloacicola, Strigea spp.) were located in the walls of the mouth cavity and gastrointestinal tract. The absence of substantial infections of internal organs and better infection rate correlation with host weight in E. recurvatum suggests that this echinostome may have little effect on its host's health.

Fig. 6. Percentages of trematode larvae located in subcutaneous tissues and visual organs (shades of red), and in walls of gastrointestinal organs (shades of green) of Pelophylax spp. post-metamorphs.
Opposite trends with lower infection rates in larger hosts were found in several diplostomid (S. strigis, T. exavata) metacercaria infections. There is no good evidence for self-clearance from well-established diplostomid infections in post-metamorphic amphibians (e.g. Rohr et al., Reference Rohr, Raffel, Sessions, Heatwole and Wilkinson2009; Raffel et al., Reference Raffel, Lloyd-Smith, Sessions, Hudson and Rohr2011; Poulin & Lagrue, Reference Poulin and Lagrue2015), yet latter larval stages may eliminate plagiorchiid metacercaria due to some unknown mechanism (Holland, Reference Holland2009). Strigeid infections may cause amphibian population decline (Sinsch et al., Reference Sinsch, Kaschek and Wiebe2018), and lower infection rates in both diplostomids in larger frogs could be explained by a parasite-induced host mortality (Loot et al., Reference Loot, Lek, Dejean and Guegan2001). In our study, most T. excavata metacercaria were found in the brain and spinal cord (supplementary table S2), which may inhibit the host's physical reaction to threats and facilitate T. excavata transmission to its definitive host – the white stork (Ciconia ciconia) (Sitko et al., Reference Sitko, Faltýnková and Scholz2006; Girisgin et al., Reference Girisgin, Birlik, Senlik and Yildirimhan2017) – in that way, fitting the optimal virulence strategy (Cressler et al., Reference Cressler, McLeod, Rozins, Van Den Hoogen and Day2016).
Waterbody size did affect helminth abundances but not their species richness in our study. The nematode O. filiformis is also common in sand lizards (Lacerta agilis) from dry terrestrial habitats (Kirillova et al., Reference Kirillova, Kirillov, Shchenkov and Chikhlyaev2020). It infects hosts through ingestion of its larvae with terrestrial food (Chikhlyaev et al., Reference Chikhlyaev, Ruchin and Fayzulin2019b) and its higher infection rates in frogs from smaller waterbodies could be caused by higher proportions of terrestrial prey items in such habitats (relevant studies on waterbody-size-specificity of their diets are absent). Trematode ontogenetic stages had opposite trends to waterbody size. Overall adult trematode infections and also infections of adults of two plagiorchiid species (P. claviger, P. medians) were higher in larger waterbodies, which could be caused by richer arthropod food resources (Heino, Reference Heino2009) and possibly higher intake rates of occasional aquatic prey by mainly onshore-feeding Pelophylax spp. frogs. Both these plagiorchiids are intermediate-host generalists, having metacercariae in an extremely wide range of aquatic arthropods, with frogs being definitive hosts (Sudarikov et al., Reference Sudarikov, Shigin, Kurochkin, Lomakin, Stenko and Yurlova2002). There are hemimetabolous insects (Plecoptera, Ephemeroptera, Odonata), which pass metacercariae into the imago stage (Mariluan et al., Reference Mariluan, Viozzi and Albarino2012) and are potentially better vectors for trematode transfer to onshore-feeding hosts, but their imago stages are rare in Pelophylax spp. diets (Tyler, Reference Toledo and Fried1958; Balint et al., Reference Balint, Citrea, Memetea, Jurij and Condure2008; Paunovic et al., Reference Paunovic, Bjelic-Cabrilo and Simic2010). Interestingly, infections of several plagiorchiids (Gorgodera varsoviensis, S. similis), having a second intermediate stage mainly in hemimetabolous Odonata (Sudarikov et al., Reference Sudarikov, Shigin, Kurochkin, Lomakin, Stenko and Yurlova2002), had no correlation with the waterbody area in our study.
First intermediate hosts for studied trematode communities are always gastropod snails, from which emerge free-swimming stages aiming to invade the next host (Galaktionov & Dobrovolskij, Reference Galaktionov and Dobrovolskij2003). In water they may reach a biomass of more than 150 mg per m3 (Preston et al., Reference Preston, Orlofske, Lambden and Johnson2013). The smallest waterbodies of our study had an area of 0.01 ha, which is the pond size with the highest larval amphibian densities (Semlitsch et al., Reference Semlitsch, Peterman, Anderson, Drake and Ousterhout2015). Elevated host densities result in increased cercariae and metacercariae prevalence in snails (Zimmermann et al., Reference Zell, Rohner, Thürig and Stadelmann2016), and high tadpole and frog densities are known to be positive for trematodes (Hartson et al., Reference Hartson, Orlofske, Melin, Dillon and Johnson2011). Hence, higher levels of larval trematode infections in amphibians from the smaller waterbodies of our study could be caused by higher host (snail, tadpole or both) densities that increase chances for short-living, free-moving miracidium and cercaria stages to meet their hosts. Trematode, especially diplostomid, larval infections may increase amphibian mortality (Sinsch et al., Reference Sinsch, Kaschek and Wiebe2018) and the use of small waterbodies by amphibians could be a trade-off between benefits from getting a breeding site free from fish predators that eradicate their larval stages (Hartel et al., Reference Hartel, Nemes, Cogălniceanu, Öllerer, Schweiger, Moga and Demeter2007; Kloskowski, Reference Kloskowski2011) and elevated risks of diseases caused by parasite infections.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X2100047X
Financial support
This research was partly supported by the project ‘Pond aquaculture production and ecosystem service innovative research with modelling of the climate impact to tackle horizontal challenges and improve aquaculture sustainability governance in Latvia’ (lzp-2020/2-0070) financed by Fundamental and Applied Research Projects (FLPP). It was also partly supported by the Joint Latvian–Ukrainian project ‘The ecological and biological triggers of expansion of the invasive fish, Chinese sleeper (Perccottus glenii), in Eastern Europe’ co-financed by the State Education Development Agency of Latvia (project number LV-UA/2018/6) and the Ministry of Science and Education of Ukraine (project number 0119U101806).
Conflicts of interest
None.
Ethical standards
Special permission for the collecting, euthanasia and study for scientific purpose was approved by the Latvian authority – Nature Conservation Agency of Latvia (permission numbers 26/2017-E, 14/2018-E, 21/2019-E and 19/2020-E).