Introduction
Parasite distribution patterns are shaped by a suite of abiotic and biotic factors, which directly and/or indirectly influence both parasites and their hosts (Ostfeld et al., Reference Ostfeld, Glass and Keesing2005). In lotic-dominated freshwater ecosystems (i.e. rivers, streams), the combined influences of the unidirectional water flow and the mobility of the most mobile host are considered to be primary drivers structuring the distribution of parasites, especially those with complex life cycles (Blasco-Costa et al., Reference Blasco-Costa, Koehler, Martin and Poulin2013; Salgado-Maldonado et al., Reference Salgado-Maldonado, Novelo-turcotte, Vazquez, Caspeta-mandujano, Quiroz-Martinez and Favila2014). This often results in increased parasite richness and abundance in a downstream direction. However, the relative importance of such drivers in structuring parasite communities of catchments dominated by lentic environments (i.e. lakes, ponds) is poorly understood, as lentic environments are seldom compared along longitudinal gradients (i.e. upper to lower catchment, although see Valtonen et al., Reference Valtonen, Holmes and Koskivaara1997; Poulin and Valtonen, Reference Poulin and Valtonen2002).
Abiotic stability and persistence, both physical and chemical, vary considerably between lentic and lotic ecosystems (Jackson et al., Reference Jackson, Peres-Neto and Olden2001), and thus the habitat available to both parasites and their hosts. The constant unidirectional water flow in lotic systems provides a mechanism for both free-living parasitic stages and infected hosts to disperse passively downstream, thus creating an environmental gradient of increasing infection from the upper to lower catchment (Blasco-Costa et al., Reference Blasco-Costa, Koehler, Martin and Poulin2013). In contrast, standing waters provide limited opportunities for passive dispersal. Host mobility may play a greater role in determining how lentic-dominated catchment parasite communities are structured, as parasites utilizing a combination of aquatic and terrestrial hosts (e.g. birds, mammals; allogenic life cycle) have greater capabilities for dispersal than parasites remaining in aquatic environments throughout their life cycle (autogenic; Esch et al., Reference Esch, Kennedy, Bush and Aho1988).
Understanding the spatial structure of parasites and their hosts at a catchment scale has become increasingly important as researchers attempt to quantify the response of freshwater ecosystems to ever increasing environmental perturbations (e.g. Hofmann et al., Reference Hofmann, Blasco-Costa, Knudsen, Matthaei, Valois and Lange2016). Whilst freshwater ecosystems are increasingly threatened by broad-scale stressors directly associated with climate change (e.g. reduced dissolved oxygen, increasing water temperature; Heino et al., Reference Heino, Virkkala and Toivonen2009; Woodward et al., Reference Woodward, Perkins and Brown2010), anthropogenic impacts on host–parasite interactions may be localized to the catchment or sub-catchment scale (e.g. point-source pollution, water impoundment; Morley, Reference Morley2007; Oros and Hanzelová, Reference Oros and Hanzelová2009; Kelly et al., Reference Kelly, Poulin, Tompkins and Townsend2010). Regardless of how such environmental perturbations arise, the absence of a priori knowledge in relation to host–parasite distribution in lentic-dominated catchments poses difficulties for quantifying parasite community impacts and recovery.
In this study we examined parasite distribution patterns in a lentic-dominated catchment in northern Norway, characterized by lake-specific populations of non-migratory Arctic charr Salvelinus alpinus (Præbel and Knudsen, Reference Præbel and Knudsen2012). This well-studied, cold-water specialist fish has diverse parasite assemblages (>20 parasite species in Norwegian freshwaters; Sterud, Reference Sterud1999), which differ greatly between populations and morpho-types (e.g. Skoglund et al., Reference Skoglund, Siwertsson, Amundsen and Knudsen2015; Siwertsson et al., Reference Siwertsson, Refsnes, Frainer, Amundsen and Knudsen2016), thus making Arctic charr an excellent candidate to test catchment-scale parasite distribution hypotheses. In particular, we assessed charr–parasite distribution baselines in a watercourse consisting of a series of oligotrophic lakes at different altitudes. The catchment's potential resilience to parasite distribution against environmental disturbances was evaluated immediately prior to the application of rotenone, a pesticide highly toxic to freshwater fish, during a regional-scale eradication programme targeted towards the invasive salmon ectoparasite Gyrodactylus salaris (Hanssen, Reference Hanssen2013).
The main aim of this study was to assess the generality of lotic-derived catchment-scale parasite assemblage patterns in lentic environments. First, we tested the overall hypothesis that parasite diversity increases in a downstream direction. Second, we assessed whether the mobility of the most mobile host determined species-specific parasite distribution patterns among lakes, independent of the upstream–downstream gradient. Specifically, we hypothesized that allogenic parasite taxa (i.e. parasites that utilized piscivorous birds as definitive hosts) would be more uniformly distributed within the catchment, whereas autogenic parasite taxa (i.e. non-migratory charr as definitive hosts) would have more restricted distributions. We also hypothesized that abundance patterns of allogenic parasites would be independent of the relative position of each lake in the catchment, whereas autogenic parasites would become increasingly abundant in a downstream direction (Blasco-Costa et al., Reference Blasco-Costa, Koehler, Martin and Poulin2013).
Materials and methods
Study area
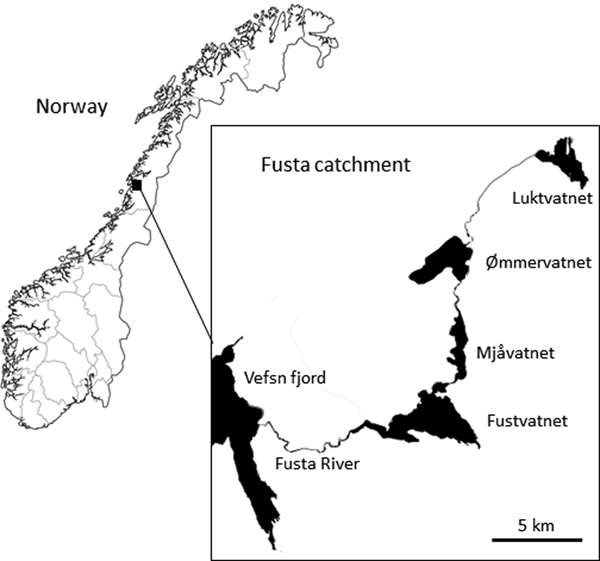
The Fusta catchment (544 km2), Nordland County, northern Norway comprises a series of linearly connected oligotrophic lakes: Luktvatnet (LV; 3.8 km2, 137 m above sea level (masl)), Ømmervatnet (ØV; 5.6 km2, 42 masl), Mjåvatnet (MV; 2.6 km2, 39 masl) and Fustvatnet (FV; 10.6 km2, 39 masl) (table 1, fig. 1). The uppermost lake, Luktvatnet, is connected to Ømmervatnet by the river Hattelva (8.0 km), with a small waterfall impeding upstream fish migration. Lakes Ømmervatnet and Mjåvatnet are connected by the river Straumanelva (1.8 km), whereas lakes Mjåvatnet and Fustvatnet are continuous apart from a short headland. Fustvatnet is connected to the Vefsn fjord by the Fusta river (8.5 km, total catchment length 56 km), where between 1880 and 1992 a fish ladder facilitated upstream migration of anadromous fish beyond a 10 m high waterfall on the river. The lower lakes (ØV, FV) are ice covered for approximately 5–6 months of the year, with the higher altitude Luktvatnet covered for an additional 4–5 weeks (Å. Kvambekk, pers. comm.; unpublished database of the Norwegian Water Resources and Energy Directorate). The dominant catchment land use is native forest (pine/spruce; >80%), although there is a gradual downstream increase in modified agricultural land (LV, 2%; ØV, 10%; FV, 15%; NVE, 2015).

Fig. 1. Lakes Luktvatnet, Ømmervatnet, Mjåvatnet and Fustvatnet of the Fusta catchment, Nordland County, Norway.
Table 1. Characteristics of Arctic charr and the lakes from which they were sampled in the Fusta catchment, Nordland, Norway.
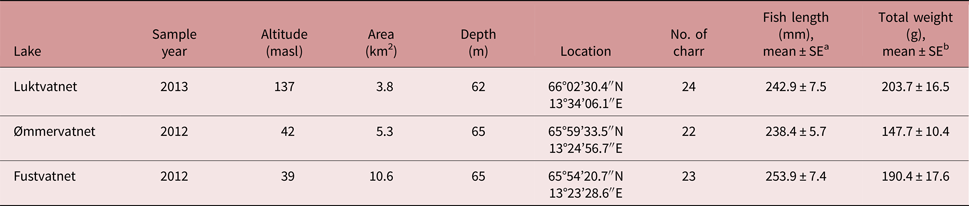
a No difference in fork length among lakes; one-way ANOVA F 2,66 = 1.28, P = 0.286
b Total weight differed between Luktvatnet and Ømmervatnet; one-way ANOVA F 2,66 = 3.59, P = 0.033; Tukey HSD
Fish communities in all lakes of the Fusta catchment consist primarily of Arctic charr, brown trout Salmo trutta, and three-spined stickleback Gasterosteus aculeatus. European eel Anguilla anguilla is rare in the system. Earlier, anadromous populations of Atlantic salmon Salmo salar and sea trout S. trutta could enter the lakes via a fish ladder in the Fusta river, but the ladder was closed in 1992 (Sæter, Reference Sæter1995).
Fish sampling
Arctic charr was selected as the focal fish species to assess parasite distribution patterns in a lentic-dominated catchment; charr have greater parasite diversity than other sympatric fish species (Knudsen et al., Reference Knudsen, Amundsen, Nilsen, Kristoffersen and Klemetsen2008; Kristmundsson and Richter, Reference Kristmundsson and Richter2009), and are represented by non-migratory populations in each lake in the study catchment (Præbel and Knudsen, Reference Præbel and Knudsen2012), thus providing an ideal system to test the influence of definitive host mobility on parasite distribution. To reduce potential variability in parasite assemblages as a result of differences in both diet and habitat associated with Arctic charr morph types (Skoglund et al., Reference Skoglund, Siwertsson, Amundsen and Knudsen2015), this study examined the littoral spawning morph only. To reduce the potential effects of host age on parasite acquisition (Poulin, Reference Poulin2000), we primarily selected fish with fork length of 200–250 mm. Additionally, to reduce the total number of fish used in this research, we utilized specimens collected during a rotenone application at Ømmervatnet (n = 24) and Fustvatnet (n = 23) in October 2012, and by gill-netting lake margins of Luktvatnet (n = 24) in October 2013. Gill-netted fish were euthanized following the strict codes of practice in force in Europe and frozen at −20°C until parasitological examination. No approval from Institutional Animal Care and Use Committee (IACUC) or ethics committee was necessary. Mjåvatnet was excluded from our study as this shallow lake supported a very limited Arctic charr population (fig. 1).
Parasite collection and identification
Each fish was thawed, measured (fork length; mm) and weighed (g), before external surfaces (e.g. fins, gill opercula) were examined macroscopically for ectoparasites. The alimentary tract, eyes and gills were removed and systematically screened for parasites. Trematode eye flukes were identified to genus level only (i.e. Diplostomum sp. or Tylodelphys sp.), however previous research suggests the simultaneous occurrence of multiple lineages and/or species from each genus is likely in each Arctic charr host (e.g. Blasco-Costa et al., Reference Blasco-Costa, Faltýnková, Georgieva, Skírnisson, Scholz and Kostadinova2014, Reference Blasco-Costa, Poulin and Presswell2017).
Data analyses
Component community (i.e. all parasites in the sampled Arctic charr in each lake, sensu Bush et al., Reference Bush, Lafferty, Lotz and Shostak1997) differences in parasite assemblages among lakes were assessed by calculating the taxa richness (total number of parasite taxa S) and Jaccard's index of community composition similarity (SJ = c/(a + b + c)), where a and b represent the number of taxa in lakes a and b, and c is the number of taxa common to both lakes. To assess infracommunity (i.e. all parasites in a single charr; Bush et al., Reference Bush, Lafferty, Lotz and Shostak1997) differences among lakes, we also calculated the number of taxa S, total abundance N, taxa diversity (Margalef's DMg; Clifford and Stephenson, Reference Clifford and Stephenson1975) and evenness (Berger–Parker index d; Berger and Parker, Reference Berger and Parker1970) per charr. Although not frequently used in parasite ecology, the Margalef's diversity index provides stronger discriminatory abilities than other more commonly used indices (e.g. Shannon) by incorporating both individual and species abundance (Magurran, Reference Magurran2003).
All statistical analyses were computed using R v. 3.3.2 (R Development Core Team, 2016). Differences in number of taxa S, total abundance N, taxa diversity D Mg and evenness d per charr among lakes were compared using separate general linear models (nlme::glm, version 3.1–131; Pinheiro et al., Reference Pinheiro, Bates, DebRoy, Sarkar and R Core2017). Differences in the abundance of each parasite taxon per charr among lakes were examined using a series of generalized linear models, with the exception of rare taxa (Contracaecum sp., Philonema oncorhynchi, Proteocephalus sp., Schistocephalus solidus, Tetraonchus sp.), which were excluded from further analysis. Models were fitted with a quasi-Poisson distribution and log-link function to account for over-dispersion, with the exception of D. sagittata, which was fitted with a Poisson distribution and log-link function. To account for the potential bias caused by variation in host age, we included fish length (centred on the mean and scaled by one standard deviation) as a continuous fixed factor in all general and generalized linear models. Contrast analyses were constructed for each model by varying the base lake (intercept) to assess differences between lake pairs. Only significant contrasts are presented in the results.
Results
Component community
Fifteen parasite taxa (4 allogenic AL, 11 autogenic AU) were identified from Arctic charr populations in the Fusta catchment, with only small differences in taxa richness (11–14 taxa) and community composition observed between lakes (LV–ØV SJ = 0.29, LV–FV SJ = 0.29, ØV–FV SJ = 0.33; table 2). However, most parasites (12 taxa) were generalist taxa also known to infect brown trout, with only three Arctic charr-specialist species present (Eubothrium salvelini, Salmincola edwardsii, P. oncorhynchi). All four allogenic parasites were present in all lakes: metacercarial eye flukes of the trematodes Diplostomum sp. and Tylodelphys sp. and the larval cestodes Diphyllobothrium dendriticum and D. ditremum; whereas minor inter-lake differences in the composition and number of autogenic taxa reflected variation in the presence of monogenean (Discocotyle sagittata, Tetraonchus sp.) and nematode taxa (Contracaecum sp., P. oncorhynchi, Pseudocapillaria sp.; S = 7 LV, 10 ØV, 8 FV; table 2). Schistocephalus solidus, an allogenic parasite acquired from consuming infected stickleback (second intermediate host), was noted in the stomach contents of charr at low prevalence and abundance throughout the catchment (table 2). In all lakes, trematodes Diplostomum sp. and Phyllodistomum umblae were the most common allogenic and autogenic taxa, representing 49–79% and 80–97% of individuals at each lake, respectively.
Table 2. Life cycle, prevalence (%) and abundance (± SE) of Arctic charr parasites (AU, autogenic; AL, allogenic) in lakes of the Fusta catchment, Nordland, Norway.
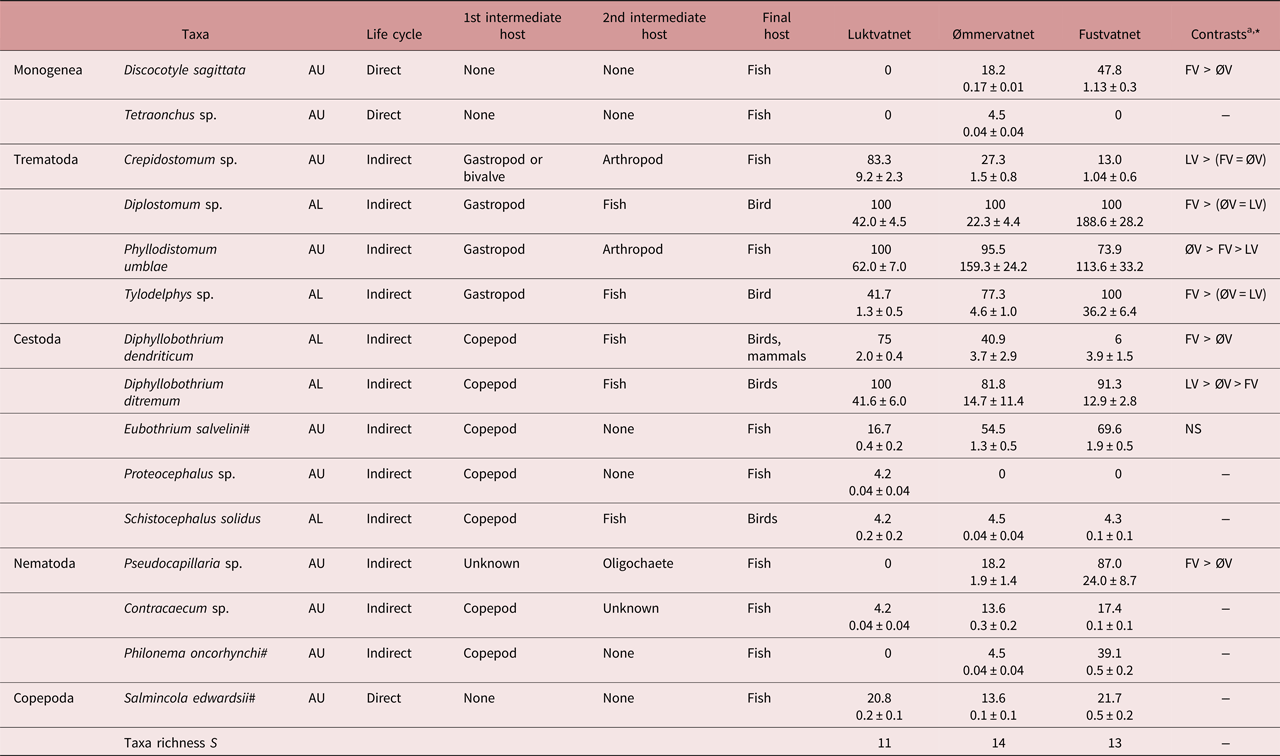
a Summary of inter-lake contrast analyses; see supplementary table S2 for details
* P < 0.05, NS – not significant
Infracommunities
Fustvatnet charr (lower catchment) harboured the highest number of parasite taxa and total infracommunity abundance, in addition to higher abundances of allogenic parasites than other lakes (tables 3 and S1). In terms of autogenic parasites, charr from both Fustvatnet and Ømmervatnet harboured more individual parasites than the upper catchment lake, Luktvatnet (tables 3 and S1). Taxa evenness (Berger–Parker), but not diversity (Margalef's), differed between all lakes (LMMLake: χ2 = 75.3, P < 0.005; Tukey HSD: all P < 0.05; ØV > FV > LV), with P. umblae and Diplostomum sp. dominating the infracommunities of Ømmervatnet and Fustvatnet, respectively (table 3, fig. 2).

Fig. 2. Parasite abundance in lakes Luktvatnet (LV), Ømmervatnet (ØV) and Fustvatnet (FV), Fusta catchment, Nordland County, Norway. Open circles indicate allogenic taxa, closed circles indicate autogenic taxa, and error bars indicate standard error. Significant differences between lakes are indicated by capital letters (P < 0.05; supplementary table S2).
Table 3. Inter-lake differences in parasite infracommunity diversity characteristics (mean ± standard error) from Arctic charr of the Fusta catchment, Nordland, Norway.
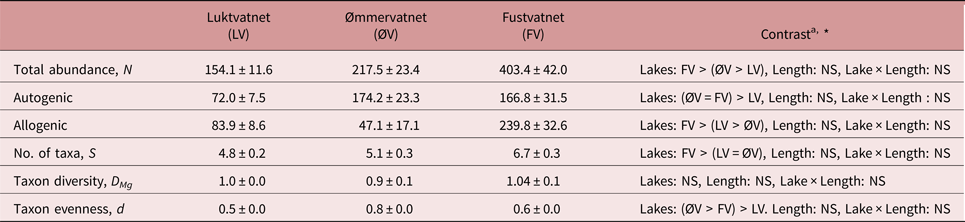
a Summary of inter-lake contrast analyses; see supplementary table S1 for details
* P < 0.05, NS – not significant
Both autogenic and allogenic parasites showed taxon-specific prevalence patterns: increasing (e.g. AU - D. sagittata, AL - Tylodelphys sp.), decreasing (e.g. AU - Crepidostomum sp., AL - D. dendriticum) or remaining unchanged along the catchment gradient (e.g. AU - S. edwardsii, AL - Diplostomum sp.; table 2). However, for most parasites (7/9 taxa), abundance increased from the upper to lower catchment and showed greatest differences between Luktvatnet and Fustvatnet (fig. 2, table S2). For example, the allogenic trematode eye fluke Tylodelphys sp. demonstrated a 10-fold increase in abundance between Luktvatnet and Fustvatnet (fig. 2). Autogenic Crepidostomum sp. and allogenic D. ditremum were the two exceptions to the general trend, occurring in highest abundance in the upper catchment lake, Luktvatnet (table S2).
Discussion
Catchment-wide parasite abundance and distribution patterns in this lentic-dominated system were in accordance with those reported for lotic systems. We found good support for the prediction of increasing parasite abundance in a downstream direction at both the infracommunity and taxa levels. We also found strong support for the hypothesis that the mobility of the most mobile host determines taxon-specific parasite distribution patterns, with all four allogenic taxa (utilizing piscivorous birds as final hosts) present throughout the entire catchment, whereas their autogenic counterparts (charr as final hosts) demonstrated restricted distributions.
Catchment-scale parasite abundance patterns driven by longitudinal or local factors have been identified to some extent in lotic systems (Blasco-Costa et al., Reference Blasco-Costa, Koehler, Martin and Poulin2013; Salgado-Maldonado et al., Reference Salgado-Maldonado, Novelo-turcotte, Vazquez, Caspeta-mandujano, Quiroz-Martinez and Favila2014), with recent work by Blanar et al. (Reference Blanar, Hewitt, McMaster, Kirk, Wang, Norwood and Marcogliese2016) suggesting that localized processes (e.g. habitat quality, land use) may play even greater roles in structuring fish–parasite communities than large-scale (e.g. distance decay; Poulin, Reference Poulin2003) or longitudinal influences (i.e. the river continuum concept; Vannote et al., Reference Vannote, Minshall, Cummins, Sedell and Cushing1980). Here too in the lentic-dominated Fusta catchment, abundance patterns may be driven by forces of unidirectional flow transporting infected hosts and/or free-living parasitic stages, and also by the localized increases in agricultural land use (2–15%; NVE, 2015) from the upper to lower catchment sufficient to create a gradient of increasing lake productivity. This coupled with existing climatic gradients enabling lakes in the lower catchment to attain earlier and longer ice-free periods, in addition to reaching higher water temperatures, has the potential to generate localized differences in the abundance of both parasites and their hosts (Hakalahti et al., Reference Hakalahti, Karvonen and Valtonen2006). Increasing water temperature, for example, may enhance trematode populations directly by enhancing egg development and cercariae release from snail intermediate hosts, in addition to increasing the reproduction rate of uninfected snails (Paull and Johnson, Reference Paull and Johnson2011).
It is important to note, however, that taxon-specific variation in parasite abundance may occur at a range of spatial scales, even for parasites sharing the same hosts in their life cycles. At large spatial scales (i.e. latitudes, between islands), parasite abundance may be non-deterministic (e.g. Blasco-Costa et al., Reference Blasco-Costa, Rouco and Poulin2015), and even at mid-to-large spatial scales (i.e. regional, inter-catchment), where heterogeneity is lower, spatial covariation in abundance has been detected (e.g. Lagrue and Poulin, Reference Lagrue and Poulin2015). In particular, Lagrue and Poulin (Reference Lagrue and Poulin2015) demonstrated that although parasite taxa that share all host life stages show greatest similarity in spatial abundance, parasites sharing two out of three host life stages do not necessarily show spatial similarity. Our observations in the Fusta catchment support this theory, with high similarity in Diplostomum sp. and Tylodelphys sp. spatial abundance trends (gastropod – fish – bird) in comparison to Crepidostomum sp. (gastropod or bivalve – arthropod – fish) and D. ditremum (copepod – fish – bird) for example. Such spatial variations in parasite abundance are likely to be further promoted by differences in local food webs (Marcogliese et al., Reference Marcogliese, Gendron, Plante, Fournier and Cyr2006), with Bérubé and Curtis (Reference Bérubé and Curtis1986) demonstrating how inter-lake differences in benthic invertebrate availability influenced D. ditremum burdens in Arctic charr. Although invertebrate communities were not assessed among our study lakes, the majority of charr parasites were trophically transmitted via the consumption of copepods or macroinvertebrates, thus the longitudinal gradient of decreasing D. ditremum abundance probably reflects a decreasing proportion of copepods (D. ditremum first intermediate host) both in the invertebrate community and charr diet.
In contrast to parasite abundance patterns, clear downstream gradients of parasite community diversity were not observed in the Fusta catchment. Component community taxa richness showed little difference between lakes, probably because of the tendency for the substitution rather than accumulation of parasite taxa along the catchment (e.g. cestode Proteocephalus sp. – LV only, monogenean Discocotyle sagittata – ØV & FV only), and to a lesser extent, the presence of widespread allogenic taxa with distributions independent of downstream water movement. Although infracommunity taxon richness suggested greater taxa richness may occur in the lower catchment, this was not supported by taxa diversity measures when both the individual and taxa abundance were accounted for. Although differences in lake size have the potential to influence parasite species richness (Kennedy, Reference Kennedy1978), it is likely that the similarity in depth (62–65 m) among the large lakes in the Fusta catchment (3.8–10.6 km2) minimizes this effect (Marcogliese and Cone, Reference Marcogliese and Cone1991).
The absence of parasite diversity gradients may also be attributed to the majority of autogenic taxa being generalist parasites known to infect brown trout; the migratory (Præbel and Knudsen, Reference Præbel and Knudsen2012) and numerically dominant salmonid species of the Fusta catchment (charr : trout ratio: LV 1 : 1.1, ØV 1 : 7.3, FV 1 : 3.4; Hanssen, Reference Hanssen2013). Although parasite dynamics are strongly linked to the relative abundance of their hosts (Arneberg et al., Reference Arneberg, Skorping, Grenfell and Read1998; Paterson et al., Reference Paterson, Rauque, Fernandez, Townsend, Poulin and Tompkins2013), this abundant salmonid species may not be exclusively responsible for the erosion of the autogenic parasite community structure among lakes. For example, autogenic-generalist taxa (e.g. Proteocephalus sp., Psuedocapillaria sp.) were more patchily distributed in the catchment than autogenic-specialist taxa of charr (e.g. Eubothrium salvelini, Salmincola edwardsii). Previous comparisons between sympatric trout and charr parasite communities have also demonstrated that charr are often the dominant host, in terms of parasite prevalence, abundance and species richness (e.g. Knudsen et al., Reference Knudsen, Amundsen, Nilsen, Kristoffersen and Klemetsen2008, Kristmundsson and Richter, Reference Kristmundsson and Richter2009), as a result of greater exposure to infectious stages through their wider habitat and dietary niches (Eloranta et al., Reference Eloranta, Knudsen and Amundsen2013), coupled with greater host–parasite compatibility.
Furthermore, there is little evidence to suggest differences in sample year (2013 vs 2012) nor fish sampling methods (gill netting vs rotenone) between Luktvatnet and the lower lakes were responsible for the observed parasite distribution patterns. Although seasonal variation in fish–parasite communities occurs in Norwegian lakes (e.g. Amundsen et al., Reference Amundsen, Knudsen, Kuris and Kristoffersen2003), inter-annual variability tends to be low (Paterson, unpublished; Kuhn et al., Reference Kuhn, Knudsen, Kristoffersen, Primicerio and Amundsen2016). Rotenone, on the other hand, is detrimental to fish ectoparasite populations via indirect effects on host mortality (e.g. Fukami et al., Reference Fukami, Shishido, Fukunaga and Casida1969), and experimental studies suggest rotenone may have anthelmintic properties towards nematodes (Kotze et al., Reference Kotze, Dobson and Chandler2006); however, the direct impacts of rotenone on fish parasites are unknown. Thus, we cannot exclude the possibility of rotenone-induced reductions in ectoparasite abundance in lakes Ømmervatnet and Fustvatnet, although influences on the parasitic copepod S. edwardsii are likely to be minimal given its securely imbedded bulla structure. Furthermore, we note that both the observed composition and abundance of parasite taxa in charr populations of the Fusta catchment lakes are similar to those of other northern Norwegian lakes (Kennedy, Reference Kennedy1977; Amundsen et al., Reference Amundsen, Kristoffersen, Knudsen and Klemetsen1997; Knudsen et al., Reference Knudsen, Kristoffersen and Amundsen1997), and thus the effect of the rotenone fish sampling method, if present, should be minimal.
By assessing parasite distribution patterns in the Fusta catchment, our study sheds light on the catchment's potential resilience against environmental disturbances (i.e. the planned salmonid eradication). Although Arctic charr specialist parasites and communities are generally stable through time (Amundsen et al., Reference Amundsen, Kristoffersen, Knudsen and Klemetsen1997; Knudsen et al., Reference Knudsen, Primicerio, Amundsen and Klemetsen2010; Kuhn et al., Reference Kuhn, Knudsen, Kristoffersen, Primicerio and Amundsen2016), they may change as a result of severe anthropogenic disturbances to salmonid populations (Amundsen and Kristoffersen, Reference Amundsen and Kristoffersen1990; Knudsen et al., Reference Knudsen, Amundsen and Klemetsen2002). Regions that avoid environmental perturbations are considered to be important reservoirs of natural propagules to facilitate the recolonization of biotic assemblages (Tonkin et al., Reference Tonkin, Stoll, Sundermann and Haase2014), a feature that may be particularly important for autogenic parasite species with limited inter-catchment dispersal capabilities. However, our assessment of catchment-wide component communities suggests that lakes Ømmervatnet and Fustvatnet may experience long-term reductions in parasite diversity after the rotenone treatment programme. Allogenic taxa with high colonization abilities (i.e. Diplostomum sp., Tylodelphys sp., Diphyllobothrium spp.) would be expected to re-establish throughout the catchment. In contrast, at least four autogenic taxa (i.e. D. sagittata, Tetraonchus sp., Pseudocapillaria sp., P. oncorhynchi) may be at risk of eradication, unless sympatric populations of brown trout or three-spined stickleback in Luktvatnet act as reservoir hosts (e.g. Hartvigsen and Halvorsen, Reference Hartvigsen and Halvorsen1993; Braicovich et al., Reference Braicovich, Kuhn, Amundsen and Marcogliese2016). Temporary impacts on Arctic charr-specialist parasite taxa (i.e. E. salvelini, S. edwardsii) would also be anticipated, although mitigated by the gradual downstream recolonization to treated lakes.
In summary, our study demonstrates the generality of catchment-wide parasite abundance and distribution patterns irrespective of the dominant habitat type. Furthermore, this study suggests that longitudinal gradients in parasite abundance may still arise in lentic-dominated catchments, despite the reduced role of unidirectional water movement.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X18000482
Acknowledgements
Fish sampling was carried out by Mosjøen og Omegn Næringsselskap in accordance with Norwegian Veterinary Institute regulations. We thank R. Poulin and two anonymous reviewers for constructive comments on an earlier version of this manuscript.
Financial support
This study was support by the Norwegian Environment Agency and internal funding from the Norwegian Veterinary Institute. RAP was also supported by the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement (no. 663830).
Conflict of interest
None.
Ethical standards
All fish were killed following the strict codes of practice in force in Europe. No approval from Institutional Animal Care and Use Committee (IACUC) or ethics committee was necessary.