No CrossRef data available.
Article contents
Transition of buoyancy-driven flow from an axisymmetric to non-axisymmetric vortex inside an evaporating sessile droplet
Published online by Cambridge University Press: 17 October 2024
Abstract
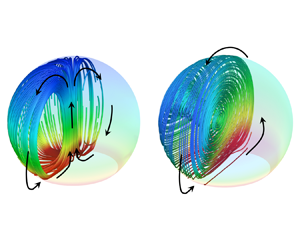
The internal flow within an evaporating sessile droplet has intriguing fluid mechanics important to various microfluidics applications. In the present study, a phenomenon is observed through numerical methods wherein the buoyancy-driven flow structure inside a droplet on a non-wetting substrate transitions from an axisymmetric toroidal vortex flow to a non-axisymmetric single vortex flow with increase in the substrate temperature. As the axisymmetric nature of the droplet flow field and evaporation characteristics are broken, the internal velocity accelerates significantly. The transition, which is attributed to a flow instability inside the droplet, is more prone to occur as the droplet volume or the contact angle increases. The onset of the flow transition is analysed as the amplification of a small perturbation, thereby establishing a correlation between the flow instability and the Rayleigh number (Ra). Specifically, when Ra exceeds some critical value, the onset of the flow transition is observed, which explains the effects of substrate temperature and droplet volume on the internal flow. Next, the influence of the droplet contact angle on the critical Ra was investigated, and the underlying reasons were analysed. Finally, we discuss the heat transfer efficiency within the droplet and analyse why the internal flow tends to transition to a non-axisymmetric flow pattern from an energy minimization perspective.
- Type
- JFM Papers
- Information
- Copyright
- © The Author(s), 2024. Published by Cambridge University Press



