Article contents
Blood flow in small tubes: quantifying the transition to the non-continuum regime
Published online by Cambridge University Press: 28 March 2013
Abstract
In small vessels blood is usually treated as a Newtonian fluid down to diameters of 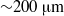 ${\sim }200~\mathrm{\mu} \mathrm{m} $. We investigate the flow of red blood cell (RBC) suspensions driven through small tubes (diameters
${\sim }200~\mathrm{\mu} \mathrm{m} $. We investigate the flow of red blood cell (RBC) suspensions driven through small tubes (diameters 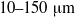 $10\text{{\ndash}} 150~\mathrm{\mu} \mathrm{m} $) in the range marking the transition from arterioles and venules to the largest capillary vessels. The results of the simulations combined with previous simulations of uniform shear flow and experimental data show that for diameters less than
$10\text{{\ndash}} 150~\mathrm{\mu} \mathrm{m} $) in the range marking the transition from arterioles and venules to the largest capillary vessels. The results of the simulations combined with previous simulations of uniform shear flow and experimental data show that for diameters less than 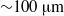 ${\sim }100~\mathrm{\mu} \mathrm{m} $ the suspension’s stress cannot be described as a continuum, even a heterogeneous one. We employ the dissipative particle dynamics (DPD) model, which has been successfully used to predict human blood bulk viscosity in homogeneous shear flow (Fedosov et al. Proc. Natl Acad. Sci. USA, vol. 108, 2011, pp. 11772–11777). In tube flow the cross-stream stress gradient induces an inhomogeneous distribution of RBCs featuring a centreline cell density peak, and a cell-free layer (CFL) next to the wall. For a neutrally buoyant suspension the imposed linear shear-stress distribution together with the differentiable velocity distribution allow the calculation of the local viscosity across the tube section. The viscosity across the section as a function of the strain rate is found to be essentially independent of tube size for the larger diameters and is determined by the local haematocrit (
${\sim }100~\mathrm{\mu} \mathrm{m} $ the suspension’s stress cannot be described as a continuum, even a heterogeneous one. We employ the dissipative particle dynamics (DPD) model, which has been successfully used to predict human blood bulk viscosity in homogeneous shear flow (Fedosov et al. Proc. Natl Acad. Sci. USA, vol. 108, 2011, pp. 11772–11777). In tube flow the cross-stream stress gradient induces an inhomogeneous distribution of RBCs featuring a centreline cell density peak, and a cell-free layer (CFL) next to the wall. For a neutrally buoyant suspension the imposed linear shear-stress distribution together with the differentiable velocity distribution allow the calculation of the local viscosity across the tube section. The viscosity across the section as a function of the strain rate is found to be essentially independent of tube size for the larger diameters and is determined by the local haematocrit (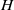 $H$) and shear rate. Other RBC properties such as asphericity, deformation, and cell-flow orientation exhibit similar dependence for the larger tube diameters. As the tube size decreases below
$H$) and shear rate. Other RBC properties such as asphericity, deformation, and cell-flow orientation exhibit similar dependence for the larger tube diameters. As the tube size decreases below 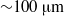 ${\sim }100~\mathrm{\mu} \mathrm{m} $ in diameter, the viscosity in the central region departs from the large-tube similarity function of the shear rate, since
${\sim }100~\mathrm{\mu} \mathrm{m} $ in diameter, the viscosity in the central region departs from the large-tube similarity function of the shear rate, since 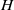 $H$ increases significantly towards the centreline. The dependence of shear stress on tube size, in addition to the expected local shear rate and local haematocrit, implies that blood flow in small tubes cannot be described as a heterogeneous continuum. Based on the analysis of the DPD simulations and on available experimental results, we propose a simple velocity-slip model that can be used in conjunction with continuum-based simulations.
$H$ increases significantly towards the centreline. The dependence of shear stress on tube size, in addition to the expected local shear rate and local haematocrit, implies that blood flow in small tubes cannot be described as a heterogeneous continuum. Based on the analysis of the DPD simulations and on available experimental results, we propose a simple velocity-slip model that can be used in conjunction with continuum-based simulations.
- Type
- Papers
- Information
- Copyright
- ©2013 Cambridge University Press
References
- 75
- Cited by


