Introduction
Birth weight is one of the fundamental principles of the infant’s future health. Previous studies have revealed a strong association between infant birth weight and perinatal outcomes or even diseases later in life. Smaller infants, such as small-for-gestational-age (SGA) and low birth weight (LBW) infants, reportedly have higher mortality rates and poorer neurodevelopmental outcomes compared with normal infants. Reference Walker and Marlow1 Infant birth weight is thought to be related to short-term neonatal and long-term prognoses. SGA and LBW infants are at a high risk of developing non-communicable diseases (NCDs) such as cardiovascular disease (CVD), diabetes (DM), and hypertension in adulthood. Reference Eriksson, Forsén, Tuomilehto, Osmond and Barker2–Reference Kanda, Murai-Takeda, Kawabe and Itoh5
Larger infants, such as large-for-gestational-age (LGA) infants and those with macrosomia, are also reportedly at risk of mortality and neonatal complications. Reference Araujo Júnior, Peixoto, Zamarian, Elito Júnior and Tonni6 An association between LGA or macrosomia and NCDs has been suggested, although this is not as common as in SGA and LBW infants. Reference Gunnarsdottir, Birgisdottir, Thorsdottir, Gudnason and Benediktsson7–Reference Mzayek, Cruickshank, Amoah, Srinivasan, Chen and Berenson10 Furthermore, macrosomia has been shown to be a risk factor for perinatal complications, such as emergency caesarean section and maternal postpartum haemorrhage. Reference Araujo Júnior, Peixoto, Zamarian, Elito Júnior and Tonni6
As described above, infant birth weight is an important factor influencing perinatal outcomes and neonatal prognosis; therefore, it is clinically important to identify the factors associated with infant birth weight. Various factors have been shown to be associated with infant birth weight; some major factors include length of the gestational period, race, socioeconomic factors, maternal lifestyle such as smoking, maternal complications such as glucose metabolism disorders, maternal pre-pregnancy body mass index (BMI), gestational weight gain, paternal BMI, and paternal height. Reference Valero De Bernabé, Soriano and Albaladejo11–Reference Magnus, Gjessing, Skrondal and Skjærven14
Although the associations between parental birth weights and infant birth weight have been reported in several studies, various parental factors known to affect infant birth weight were not considered in these studies. Reference Magnus, Gjessing, Skrondal and Skjærven14–Reference Kuzawa and Eisenberg16 For example, perinatal complications, maternal complications, and paternal socioeconomical factors were not adjusted in the statistical analysis. In addition, to the best of our knowledge, no previous studies have evaluated the association of maternal and paternal birth weight with infant birth weight in the Japanese population. This study aimed to investigate the association between maternal and paternal birth weights and infant birth weight in a large Japanese cohort considering that considered as many factors as possible.
Methods
Study design
We obtained data from the Japan Environment and Children’s Study (JECS), an ongoing nationwide birth cohort study conducted in Japan. The primary objective of the JECS is to investigate the association between environmental factors, such as chemical exposure, and children’s health and development. Pregnant women and their partners were recruited from 15 Regional Centres between January 2011 and March 2014. In the JECS, pregnant women answered the two questionnaires – the MT1 and MT2 – during pregnancy. The MT1 and MT2 questionnaires were administered in the first, second/third trimesters. Partners of pregnant women answered the FT1 questionnaire up to one month after delivery. In addition, a questionnaire – C6m – was administered six months after the delivery of their offspring. The study protocol and baseline characteristics of the participants in the JECS have been previously described. Reference Shah17,Reference Kawamoto, Nitta and Murata18 In this study, we analysed the “jecs-ta-20190930” dataset released in October 2019 by the Programme Office. A detailed baseline profile of the participants in the JECS has already been presented. Reference Michikawa, Nitta and Nakayama19
Parental birth weights
Data on parental birth weights were collected using the C6m questionnaire. With reference to previous studies, parental birth weights were categorised with 500 g intervals as follows: <2,500 g, 2,500–2,999 g, 3,000–3,499 g, 3,500–3,999 g, and ≥ 4,000 g. Reference Mattsson and Rylander15,Reference Andraweera, Dekker and Leemaqz20–Reference Lie, Wilcox and Skjærven23
Infant birth weight
The primary outcome was the infant birth weight. The reference range for Japanese infant birth weight in percentiles comprises infant birth weight, delivery week, parity (primipara or not), and infant sex. Reference Uehara, Miura, Itabashi, Fujimura and Nakamura24,Reference Kazuo, Masanori, Satoshi, Masanori, Tokinaka and Tsutomu25 Delivery week ≥ 42 weeks of gestation were excluded from the study because of a lack of reference.
Data on infant birth weight, delivery week, and sex were transcribed from medical records. Parity was transcribed from medical records. Based on the reference range of Japanese infant birth weight, infant birth weight in percentiles was classified as SGA, AGA, and LGA. SGA, AGA, and LGA infants were defined as infants with birth weights < 10th percentile, ≥10th but < 90th percentile, and ≥ 90th percentile, respectively. Infant birth weight (g) was also classified as LBW, normal birth weight, and macrosomia. Infants with LBW and macrosomia were defined as having birth weights of < 2,500 g and ≥ 4,000 g, respectively. Normal birth weight was defined as birth weight ≥ 2,500 g but ≤ 4,000 g.
Other parental variables in this study
Maternal age in the MT1 questionnaire and regions where Regional Centres existed were also provided in the dataset. Regions with Regional Centres were classified into Hokkaido, Tohoku, Kanto, Chubu, Kinki, Chugoku, Shikoku, and Kyusyu-Okinawa. Data on maternal pre-pregnancy body weight (BW), height, pre-delivery BW, parity, conception method, and number of fetuses were transcribed from medical records. The pre-pregnancy BMI was calculated based on maternal pre-pregnancy BW and height. Gestational weight gain was calculated by subtracting the pre-pregnancy BW from the BW just before delivery. Parity was categorised as primipara and multipara. Conception methods were classified into spontaneous pregnancy; non-assisted reproductive technology (ART), including ovulatory induction and artificial insemination by the husband (AIH); and ART, including in vitro fertilisation and embryo transfer (IVF-ET) and intracytoplasmic sperm injection (ICSI). Medical history of hypertension, type 1 and type 2 diabetes mellitus, kidney disorder, hyperthyroidism, hypothyroidism, systemic lupus erythematosus (SLE), and mental illness was obtained from the MT1 questionnaire. A history of antiphospholipid syndrome (APS) was also transcribed from medical records. Kidney disorders were defined as immunoglobulin A nephropathy, glomerular nephritis, and/or nephrotic syndrome. SLE and APS were combined into categories of SLE and/or APS. Mental illness was defined as depression, anxiety disorder, schizophrenia, or dysautonomia. Maternal smoking history and alcohol consumption data were obtained from both the MT1 and MT2 questionnaires. Maternal smoking status was classified as follows: never, previously did but quit before realising current pregnancy, previously did but quit after realising current pregnancy, smoking in only the MT1 questionnaire, smoking in only the MT2 questionnaire, and smoking in both the MT1 and MT2 questionnaires. Maternal alcohol consumption was classified as follows: never, quit drinking before awareness of conception, quit drinking after awareness of conception, drinking in only the MT1 questionnaire, drinking in only the MT2 questionnaire, and drinking in both the MT1 and MT2 questionnaires. Data on the highest maternal education level, obtained from the MT2 questionnaire, was classified as follows: junior high school, high school, technical junior college, technical/vocational college, associate degree, bachelor’s degree, and graduate degree (Master’s/Doctor’s). Data on hypertensive disorders of pregnancy (HDP) and gestational diabetes mellitus (GDM) were also transcribed from medical records.
The paternal age in the FT1 questionnaire was also provided in the dataset. Data on paternal height, BW, smoking status, and alcohol consumption were obtained from the FT1 questionnaire. Paternal BMI was calculated based on height and BW. Paternal smoking status in the FT1 questionnaire was classified as follows: never, previously did but quit before realising their partner’s pregnancy, previously did but quit after realising their partner’s pregnancy, and currently smoking. Paternal alcohol consumption in the FT1 questionnaire was classified as follows: never, quit drinking, and continued drinking. The highest paternal level of education, collected from the MT2 questionnaire, was classified as follows: junior high school, high school, technical junior college, technical/vocational college, associate degree, bachelor’s degree, and graduate degree (Master’s/Doctor’s). The maternal marital status obtained from the MT1 questionnaire was classified as married, unmarried, divorced, or widowed. The annual household income was collected from the MT2 questionnaire and classified as follows: <2.00, 2.00–3.99, 4.00–5.99, 6.00–7.99, 8.00–9.99, 10.00–11.99, 12.00–14.99, 15.00–19.99, and ≥ 20.00 million Japanese yen.
Statistical analysis
The parental and neonatal characteristics of the study participants were summarised using the gtsummary package of R, version 4.1.2. Reference Daniel, Michael and Margie26,27 We also used SAS software (version 9.4; SAS Institute Inc., Cary, North Carolina, USA) to perform other statistical analyses.
Continuous variables are expressed as mean (standard deviation) or median (interquartile range), as appropriate. Categorical variables are expressed as numbers (percentages).
We applied a multinomial logistic regression model to evaluate the association between parental and infant birth weights. Maternal and paternal birth weights of 3,000–3,499 g were used as the reference categories. In the analysis of the association of parental birth weight with SGA or LGA infants, AGA infants were used as the reference category. Infants with normal birth weight (i.e. birth weight ≥ 2,500 g but < 4,000 g) were set as the reference category in the analysis of the association between parental birth weight and LBW infants or infants with macrosomia.
First, parental birth weights, as categorical variables, were included in a multinomial logistic regression model. Next, a linear trend test was conducted for the association between parental birth weight categories and infant birth weight. Parental birth weight, as a continuous variable, was included in the model, and the odds ratios (ORs) per 500 g increase or decrease in the parental birth weights were calculated. Model 1 was a crude analysis. Model 2 was adjusted for regions where Regional Centres existed, marital status, annual income, infant sex, maternal variables, and paternal variables. Maternal variables included age, height, pre-pregnancy BMI, gestational weight gain, conception method, parity (primipara or not), history of diseases (hyperthyroidism, hypothyroidism, SLE and/or APS, mental illness, and kidney disorder), smoking history, alcohol consumption, and highest level of education. Paternal variables included age, height, BMI, smoking history, alcohol consumption, and highest level of education. Because a history of type 1 or 2 diabetes, GDM, and HDP was considered a potential intermediate variable with reference to previous studies, Model 3 was created by adjusting for history of type 1 or 2 diabetes, GDM, and HDP in addition to model 2. Reference Valero De Bernabé, Soriano and Albaladejo11,Reference Damm, Houshmand-Oeregaard, Kelstrup, Lauenborg, Mathiesen and Clausen28 To avoid strong multicollinearity, the categories of highest parental levels of education and annual household income were combined. Junior high school and high school were combined into a single category. Bachelor’s and graduate degrees (Master’s/Doctor’s) were also combined into one category. Annual household income was combined into the following categories: <12, 12–14.99, and ≥ 15 million Japanese yen. Improbable values of gestational weight gain (i.e. 522.6 and 687 kg), height (16 cm), and paternal BW (610 kg) were treated as missing values. In both Models 2 and 3, we conducted a multiple imputation using the Markov chain Monte Carlo simulation to complement the missing values of the independent variables. The dependent (i.e. classification of infant birth weight into percentiles and grams) and independent variables used in Model 3 were included in the construction of an imputation model. After 30 datasets were created using multiple imputations, each dataset was analysed in the same model. Finally, the 30 results were combined. The combined results are presented in this manuscript. As an additional analysis, we evaluated the association between parental birth weight and infant birth weight stratified by infant sex. A two-sided P-value<0.05 indicated statistical significance.
Results
Parental and neonatal characteristics of study participants
Figure 1 shows the flowchart of this study. The “jecs-ta-20190930” dataset contains 103,060 pregnancies. Pregnancies were excluded for the following reasons: multiple participation in the JECS (n = 5,647), divorced or widowed marital status (n = 835), paternal refusal to consent to the JECS (n = 47,505), and abortion or stillbirth (n = 332), maternal consent was withdrawal or censoring (n = 2,399), paternal consent was withdrawal or censoring (n = 419), maternal nationality was not Japan (n = 221), missing data on maternal nationality (n = 2,516), paternal nationality was not Japan (n = 179), missing data on paternal nationality (n = 124), multiple pregnancies (n = 409), missing data on maternal birth weight (n = 1,545), and improbable data on maternal birth weight (i.e. ≤3 g; n = 4), missing data on paternal birth weight (n = 2,167), improbable data on paternal birth weight (i.e. ≤72 g; n = 7), delivery week of ≥ 42 weeks of gestation (n = 104), missing data on delivery weeks (n = 62), missing data on parity(n = 1,064) and missing data on infant birth weight (n = 17). Finally, 37,504 pregnant women and their partners were analyzed.

Figure 1. Flow chart of this study.
Table 1 shows the parental and neonatal characteristics of the study participants. The number (%) of mothers with birth weights < 2,500 g and ≥ 4,000 g was 1,808 (4.8) and 855 (2.3), respectively. The number (%) of paternal birth weights < 2,500 g and ≥ 4,000 g was 1,352 (3.6) and 1,705 (4.5), respectively. The numbers (%) of SGA and LGA infants were 2,770 (7.4) and 3,837 (10.2), respectively. The numbers (%) of LBW and macrosomia infants were also 2,817 (7.5) and 306 (0.8), respectively.
Table 1. Parental and neonatal characteristics of the study participants

Continuous variables are expressed as mean (standard deviation). Categorical variables are expressed as numbers (percentages). Abbreviations: AGA, appropriate for gestational age; APS, antiphospholipid syndrome; ART, assisted reproductive technology; BMI, body mass index; GDM, gestational diabetes mellitus; HDP, hypertensive disorders of pregnancy; LGA, large for gestational age; SGA, small for gestational age; SLE, systemic lupus erythematosus.
Supplementary Tables S1 and S2 show parental and neonatal characteristics according to maternal and paternal birth weight, respectively.
Association of maternal birth weight with infant birth weight
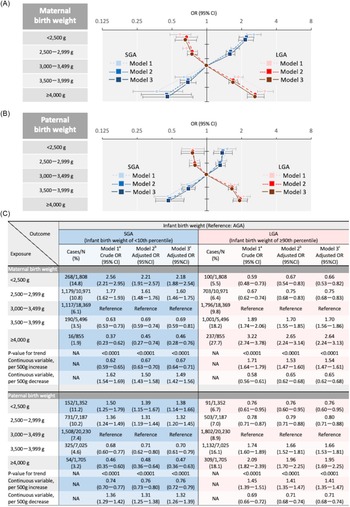
Figure 2a shows the association between maternal and infant birth weights. Linear associations were found between maternal birth weight and SGA or LGA infants in Model 2 (P < 0.0001 and < 0.0001, respectively). The adjusted odds ratio (aOR) for SGA infants per 500 g decrease in maternal birth weight was 1.50 (95% confidence interval [CI],1.43–1.58), while that for LGA infants per 500 g increase in maternal birth weight was 1.53 (95% CI, 1.47–1.60).

Figure 2. Association of parental birth weights with infant birth weight (SGA or LGA). (A) Both maternal and paternal birth weight were included in a multinomial logistic regression model. (B) Adjusted for regions where regional centres exist, marital status, annual income, infant sex, maternal variables, and paternal variables in addition to model 1. Maternal variables included age, height, pre-pregnancy BMI, gestational weight gain, conception method, parity (primipara or not), history of the following diseases (hyperthyroidism, hypothyroidism, SLE and/or APS, mental illness, and kidney disorder), smoking status, alcohol consumption, highest level of education. Paternal variables included age, height, BMI, smoking status, alcohol consumption, and highest level of education. (C) Adjusted for history of type 1 or 2 diabetes, GDM, and HDP in addition to model 2. Abbreviations: AGA, appropriate for gestational age; APS, antiphospholipid syndrome; BMI, body mass index; CI, confidence interval; GDM, gestational diabetes mellitus; HDP, hypertensive disorders of pregnancy; LGA, large for gestational age; NA, not applicable, OR, odds ratio; SGA, small for gestational age; SLE, systemic lupus erythematosus.
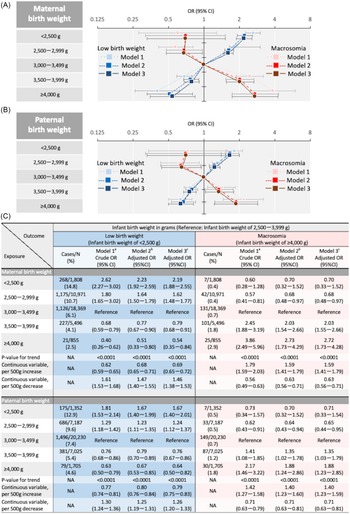
As shown in Figure 3a, the association of maternal birth weight with LBW infants or infants with macrosomia was also linear (P-values for trend were < 0.0001 and < 0.0001, respectively). The aOR for LBW infants per 500 g decrease in maternal birth weight was 1.47 (95% CI, 1.40–1.55); that for macrosomia per 500 g increase in maternal birth weight was 1.58 (95% CI, 1.41–1.79). Model 3 showed results similar to those of Model 2.

Figure 3. Association of parental birth weights with infant birth weight (Low birth weight infant or macrosomia). (A) Both maternal and paternal birth weight were included in a multinomial logistic regression model. (B) Adjusted for regions where regional centres exist, marital status, annual income, infant sex, maternal variables, and paternal variables in addition to model 1. Maternal variables included age, height, pre-pregnancy BMI, gestational weight gain, conception method, parity (primipara or not), history of the following diseases (hyperthyroidism, hypothyroidism, SLE and/or APS, mental illness, and kidney disorder), smoking status, alcohol consumption, and highest level of education. Paternal variables included age, height, BMI, smoking status, alcohol consumption, and highest level of education. (C) Adjusted for history of type 1 or 2 diabetes, GDM, and HDP in addition to model 2. Abbreviations: APS, antiphospholipid syndrome; BMI, body mass index; CI, confidence interval; not applicable, GDM, gestational diabetes mellitus; HDP, hypertensive disorders of pregnancy; OR, odds ratio; SLE, systemic lupus erythematosus.
Association of paternal birth weight with infant birth weight
Figure 2b shows the association between paternal and infant birth weights. Linear associations of paternal birth weight with SGA or LGA infants were found in Model 2 (P < 0.0001 for maternal birth weight and < 0.0001 for paternal birth weight). The aOR for SGA infants per 500 g decrease in paternal birth weights was 1.31 (95% CI, 1.25–1.38); that for LGA infants per 500 g increase in paternal birth weight was 1.41 (95% CI, 1.35–1.47).
The association of paternal birth weight with LBW infants or infants with macrosomia was also linear (P < 0.0001 for maternal birth weight and < 0.0001 for paternal birth weight; Fig. 3b). The aOR for LBW infants per 500 g decrease in paternal birth weight was 1.25 (95% CI, 1.19–1.31); that for macrosomia per 500 g increase in paternal birth weight was 1.40 (95% CI, 1.23–1.59). Model 3 showed results similar to those of Model 2.
Association of parental birth weights with infant birth weight according to infant sex
Supplementary Figures S1 and S2 illustrate the association between parental birth weight and male infant birth weight. In Model 2, the lower the parental birth weight, the higher the odds of male SGA and LBW infants (P < 0.0001 for maternal birth weight and < 0.0001 for paternal birth weight). In contrast, the higher the parental birth weight, the higher the odds of LGA and macrosomia in male infants. The results in Model 3 were used in Model 2.
Supplementary Figures S3 and S4 also illustrate the association between parental birth weight and female infant birth weight. The lower the parental birth weight, the higher the odds of female SGA and LBW infants in Model 2 (P < 0.0001 for maternal birth weight and < 0.0001 for paternal birth weight). The higher the parental birth weight, the higher the odds of female LGA infants (P < 0.0001 for maternal birth weight and < 0.0001 for paternal birth weight). Regarding macrosomia, the higher the maternal birth weight, the higher the odds of macrosomia (P = 0.001). In addition, the higher the paternal birth weight, the higher the odds of macrosomia, except for paternal birth weight < 2,500 g. Model 3 also showed similar results to Model 2.
Discussion
This study showed the associations between maternal and paternal birth weights and infant birth weight in a large Japanese cohort considering as various parental factors. Several previous studies have reported about the associations between parental birth weights and infant birth weight. Reference Magnus, Gjessing, Skrondal and Skjærven14–Reference Kuzawa and Eisenberg16 However, various parental factors known to affect infant birth weight such as perinatal complications, maternal complications, and paternal socioeconomical factors were not considered in their studies. These factors were included in the current study, and still parental birth weights were found to be associated with the offspring birth weight. In addition, the present study is the first nationwide study in Japan to examine the associations of maternal and paternal birth weights with offspring birth weight. It has been reported that there are ethnical differences in the degree of association between parental birth weights and the birth weight of their offspring. Reference Valero De Bernabé, Soriano and Albaladejo11 Therefore, the present study has a great importance because of its detailed analysis of the association between parental birth weights and infant birth weight in Japan.
This study showed that the risk of smaller infants, classified as SGA and LBW infants, was lower as parental birth weights increased, and conversely, the risk of larger infants, classified as LGA infants and macrosomia, increased as parental birth weights increased. First, the present study revealed that low maternal birth weight was a risk factor for giving birth to smaller infants, both SGA and LBW, which is consistent with numerous previous studies. Reference Hackman, Emanuel, van Belle and Daling29 Infants born to mothers with SGA are at higher risk of being SGA. Reference Mattsson and Rylander15,Reference Sepúlveda‐Martínez, Rodríguez‐López and Paz Y Miño30 Shibata et al. reported that low maternal birth weight (<2,500 g) was significantly associated with SGA and LBW infants among the Japanese population. Reference Shibata, Ogawa and Kanazawa21 Therefore, low maternal birth weight is strongly associated with the delivery of smaller infants.
Regarding paternal birth weight, we demonstrated that low paternal birth weight was a risk factor for both SGA and LBW infants. Although few studies have reported maternal birth weight, several studies have indicated that low paternal birth weight is a risk factor for LBW infants which is again consistent with our findings. Reference Damm, Houshmand-Oeregaard, Kelstrup, Lauenborg, Mathiesen and Clausen28,Reference Little31
Regarding larger infants, there is very limited evidence showing an association between parental birth weight and macrosomia or LGA infants. Several studies have reported a positive association between maternal and infant birth weight. Reference Skjærven, Wilcox, Øyen and Magnus32,Reference Agnihotri, Antonisamy, Priya, Fall and Raghupathy33 Agnihotri et al. reported a positive association between parental and infant birth weights. Reference Mutsaerts, Groen and Buiter-Van Der Meer34
These studies partly support our findings; however, they differ from the current study in terms of their sample sizes, which were larger in scale and homogeneous in race.
On the strength of our large sample size, we were able to include various adjustment items known to affect infant birth weight in the analysis; these included socioeconomic status, pre-pregnancy BMI, gestational weight gain, smoking, and HDP. Reference Valero De Bernabé, Soriano and Albaladejo11 Maternal factors that cause macrosomia, such as DM, GDM, Reference Damm, Houshmand-Oeregaard, Kelstrup, Lauenborg, Mathiesen and Clausen28 pre-pregnancy BMI, Reference Ounjaijean, Wongthanee and Kulprachakarn35 gestational weight gain, Reference Myatt and Maloyan36 and age Reference Day, Savani, Krempley, Nguyen and Kitlinska37 , were also considered in this study. Age, Reference Shah17 low socioeconomic status, Reference Oldereid, Wennerholm and Pinborg38 height Reference Damm, Houshmand-Oeregaard, Kelstrup, Lauenborg, Mathiesen and Clausen28 , and smoking Reference Damm, Houshmand-Oeregaard, Kelstrup, Lauenborg, Mathiesen and Clausen28,Reference Ounjaijean, Wongthanee and Kulprachakarn35 have been reported as paternal factors affecting LBW infants. In contrast to maternal BMI, whether there is an association between parental BMI and infant birth weight remains controversial. Reference Cox, Sharma and Evangelou39
Figure 4 is a directed acyclic graph (DAG) showed what factors influenced the parental birth weights and the infant birth weight. Even after adjusting for various factors, parental birth weight was still associated with infant birth weight in the present study. Parental birth weights are thought to be influenced by either gestational period, fetal growth (SGA of LGA), or both. Since gestational period of the parents were not available for this study, it was not possible to examine which of these influences may have been present. Previous studies have reported an association between maternal gestational period and the infant gestational period. Reference Lie, Wilcox and Skjærven23 An association between parental SGA and infant SGA has also been reported. Reference Sepúlveda‐Martínez, Rodríguez‐López and Paz Y Miño30 Thus, both gestational week and fetal growth may influence intergenerational transmission of birth weight. However, the mechanism underlying intergenerational transmission of birth weight is poorly understood.

Figure 4. Directed acyclic graph (DAG) of the associations between parental birth weights and infant birth weight.
Many of the known factors affecting gestational period and fetal growth were adjusted for in the statistical analysis in our study. Nevertheless, our study found the associations between the parental birth weights and the infant birth weight. In other words, parental factors not adjusted for in this study may have influenced the infant birth weight. First, genetic background of parents was not considered in our study. Several genes have been reported to affect preterm birth (PTB) and placental function, Reference Strauss, Romero and Gomez-Lopez40–Reference Ncube, Enquobahrie and Burke42 and may be responsible for intergenerational transmission of birth weight. Ethnic differences have been reported in the association between the maternal gestational period and the infant gestational period, Reference Cavalli and Heard43 which also supports the idea that parental genetic background influences the infant birth weight. Genetic background involves not only the genome but also the epigenome. Epigenetic change has been reported to be caused by various environmental factors, Reference Hamada and Matthews44 and many of the factors affecting the maternal epigenome have been adjusted in this study. However, it is possible that factors not adjusted for in this study may affect the epigenome. For example, steroid administration to mother with threatened preterm delivery has been suggested to affect the next generation via the epigenome. Reference Dimofski, Meyre, Dreumont and Leininger-Muller45 Second, the paternal nutritional status may also have influenced the infant birth weight. It has been reported in both humans and animals that changes in the epigenome, which is involved in lipid and glucose metabolism, are transmitted to the next generation via sperm. Reference Kuriyama, Metoki and Kikuya46 Although paternal BMI was adjusted in our study, other paternal nutritional factors were not considered in our study.
These suggest that parental birth weights may influence the infant birth weight through changes in the genome and epigenome. Future studies are expected to determine the extent to which changes in the epigenome contribute to infant birth weight of infants.
This study has several strengths. The JECS is a nationwide prospective birth cohort study with a large sample size. It covers the whole from Hokkaido in the north to Okinawa in the south. The child coverage was approximately 45% in 2013, the selected characteristics of the mothers and children were similar to that in the national survey and other birth cohort study in Japan. Reference Michikawa, Nitta and Nakayama19,Reference Kishi, Sasaki and Yoshioka47,Reference Miyake, Tanaka and Arakawa48 Specifically, maternal age, BMI, gestational weeks at delivery, PTB, birth weight, smoking status, alcohol consumption, parity, highest level of education, and annual household income were similar. Therefore, we think we can extrapolate the JECS results to the Japanese general population. Additionally, many variables, including lifestyle habits and socioeconomic factors, were considered in the statistical analysis.
However, this study has several limitations. First, information on parental birth weight was obtained using a self-administered questionnaire. Therefore, there is a possibility of misclassification and/or digit preference for parental birth weight. We could not confirm whether the study participants confirmed their Maternal and Child Health Handbooks with their own birth weight. However, in line with other studies in which maternal birth weight was collected from birth records or Maternal and Child Health Handbooks, the proportions of preterm delivery and LBW infants were higher with maternal birth weight < 2,500 g than with maternal birth weight between 3,000 and 3,499 g in this study. Reference Iwama, Obara and Ishikuro22 Therefore, we believe that this limitation did not have a critical impact on our results. Second, many participants were excluded from the analysis. As shown in the Supplementary Table S3, several variables have a statistically significant difference between the analysed group and excluded group. However, the characteristics of the parents and neonates in our study were similar to those in the other birth cohort studies in Japan. Reference Michikawa, Nitta and Nakayama19,Reference Kishi, Sasaki and Yoshioka47,Reference Miyake, Tanaka and Arakawa48 In addition, the results of the present study were similar to those of the other study. Reference Iwama, Obara and Ishikuro22 Therefore, external validity of our study would not be low. Third, as described above, it was not possible to analyse whether preterm birth or SGA (as a proxy of FGR) or both affected the results in our study because gestational period was not considered in this study. The association between parental birth weights and PTB would be investigated in the JECS. Fourth, it was not possible to analyse the contribution of each covariate to infant birth weight in because of the multiple imputation for of missing data on covariates in this study.
In conclusion, the present study showed associations between parental birth weights and infant birth weight. Thus, obtaining the birth weights of pregnant women and those of their partners as part of their medical history at prenatal check-ups could play an important role in predicting the neonates’ birth weight. Future studies are needed to elucidate the mechanism by which parental birth weight influences the infant’s birth weight.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2040174423000387.
Acknowledgments
We thank all study participants and all staff in the JECS. We would like to thank Editage (https://www.editage.com/) for their support in revising this manuscript.
Author contribution
HT, NI, and HH contributed to the conception and design of the work, interpretation of data for the work, and co-drafting of the manuscript. NI contributed to data collection and performed all statistical analyses in this study. RK, KT, NK, NS, SI, ZW, TH, MS, KS, and JS contributed to the interpretation of data and revision of the manuscript. KS, MI, TO, NT, HM, SK, TA, and NY contributed to data collection, data interpretation, and revision of the manuscript. All authors read and approved the final manuscript.
Financial support
This study was funded by the Ministry of Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the above government.
Competing interests
None.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation in Japan and with the Helsinki Declaration of 1975, as revised in 2008, and has been reviewed and approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies and the Ethics Committees of all participating institutions. Written informed consent was obtained from all the participants in the JECS.